APP, also known as amyloid-beta A4 protein, A4 or AD1, is an integral membrane protein widely expressed in many tissues including the brain, kidney, and spleens. It is known as the precursor protein which undergoes extensive post-translational modification including glycosylation, sialylation, phosphorylation, and tyrosine sulfation, as well as a number of types of proteolytic processing to form peptide fragments. The amyloid beta involved in the pathological process of Alzheimer's disease (AD) is formed through sequential cleavage of the APP.
| Basic Information of APP | |
| Protein Name | Amyloid-beta A4 protein |
| Gene Name | APP |
| Aliases | A4, AD1 |
| Organism | Homo sapiens (Human) |
| UniProt ID | P05067 |
| Transmembrane Times | 1 |
| Length (aa) | 770 |
| Sequence | MLPGLALLLLAAWTARALEVPTDGNAGLLAEPQIAMFCGRLNMHMNVQNGKWDSDPSGTKTCIDTKEGILQYCQEVYPELQITNVVEANQPVTIQNWCKRGRKQCKTHPHFVIPYRCLVGEFVSDALLVPDKCKFLHQERMDVCETHLHWHTVAKETCSEKSTNLHDYGMLLPCGIDKFRGVEFVCCPLAEESDNVDSADAEEDDSDVWWGGADTDYADGSEDKVVEVAEEEEVAEVEEEEADDDEDDEDGDEVEEEAEEPYEEATERTTSIATTTTTTTESVEEVVREVCSEQAETGPCRAMISRWYFDVTEGKCAPFFYGGCGGNRNNFDTEEYCMAVCGSAMSQSLLKTTQEPLARDPVKLPTTAASTPDAVDKYLETPGDENEHAHFQKAKERLEAKHRERMSQVMREWEEAERQAKNLPKADKKAVIQHFQEKVESLEQEAANERQQLVETHMARVEAMLNDRRRLALENYITALQAVPPRPRHVFNMLKKYVRAEQKDRQHTLKHFEHVRMVDPKKAAQIRSQVMTHLRVIYERMNQSLSLLYNVPAVAEEIQDEVDELLQKEQNYSDDVLANMISEPRISYGNDALMPSLTETKTTVELLPVNGEFSLDDLQPWHSFGADSVPANTENEVEPVDARPAADRGLTTRPGSGLTNIKTEEISEVKMDAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIATVIVITLVMLKKKQYTSIHHGVVEVDAAVTPEERHLSKMQQNGYENPTYKFFEQMQN |
APP is a cell surface receptor and transmembrane precursor protein. It cleaves to produce many peptides catalyzed by proteolytic enzymes α-, β- and γ-secretase. Some of these peptides can accelerate transcriptional activation through binding to the acetyltransferase complex APBB1/TIP60, while others form the protein basis of the amyloid plaques which are associated with the progression of AD. Some physiological studies found that APP peptide is present on the surface of neurons where it is related to neurite growth, neuronal adhesion, and axonogenesis. In vitro, the copper-metallated APP peptide induces nerve cell death directly or is enhanced through the oxidation of low-density lipoprotein-mediated by Cu2+. Besides, APP also regulates neurite outgrowth through combining with the extracellular matrix components such as collagen I and IV as well as heparin. In addition, APP also induces an AGER-dependent pathway which is involved in the p38 MAPK activation, leading to amyloid-beta peptide internalization and mitochondrial dysfunction in cultured cortical neurons. Mutations in APP gene have been indicated the association with autosomal dominant AD and cerebroarterial amyloidosis.
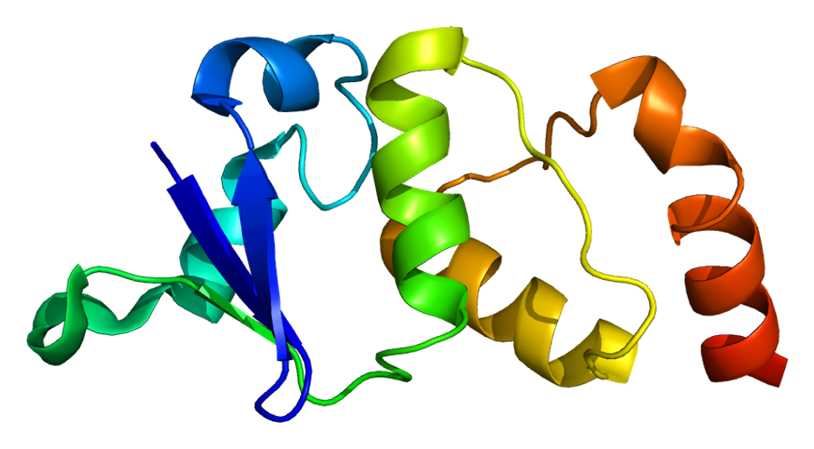 Fig .1 Available structures of APP.
Fig .1 Available structures of APP.
The study supports the hypothesis that the deficiency of APP destroys the microtubule-based axonal transport system which is a cause of AD pathology.
This review highlights the association between oxidative stress and AD and the consequences towards the Aβ peptide and surrounding molecules in terms of oxidative damage.
The investigation shows that the cytoplasmic region of APP is necessary and sufficient for the enhanced fast velocity of APP transport by kinesin-1.
The authors explain neuronal and synaptic morphological abnormalities in APPKO mice and the presence of abnormal APP expression in dystrophic neurites around amyloid deposits in AD.
The study shows that the increased APP, IL-1β, and C1QA expression and APP-expressing macrophages in periodontitis-affected gingival tissues are detected, implicating an association between periodontitis and AD pathogenesis.
To obtain the soluble and functional target protein, the versatile Magic™ membrane protein production platform in Creative Biolabs enables many flexible options, from which you can always find a better match for your particular project. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-APP antibody development services.
As a forward-looking research institute as well as a leading custom service provider in the field of membrane protein, Creative Biolabs has won a good reputation among our worldwide customers for successfully accomplishing numerous challenging projects including generation of many functional membrane proteins. Please feel free to contact us for more information.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.