AAV Capsid Modification
As a leading company in the gene therapy area, Creative Biolabs is able to offer comprehensive AAV capsid modification services for worldwide researchers, including but not limited to the site-specific modification, transcapsidation, and chimeric capsid construction. With years of experience, our scientists have further refined the tropism of AAV through numerous methods, such as genetic modification of AAV Vector, adapter mediated AAV vector targeting, etc.
Background
Among all gene therapy delivery vectors, AAV is currently the main delivery vector for treating various diseases. However, preclinical and clinical studies of AAV based gene therapy are limited by factors such as host induced antagonistic immunogenic responses, vector dose requirements, off target toxicity, and restricted tissue transduction. This is entirely attributed to the hybridity of AAV capsid to multiple tissues and its serum positivity rate. Due to these major limitations, the design of targeted AAV vectors for selective targeting of specific tissues has been based on modifying different capsid proteins and inserting non-natural amino acids. In this context, people have carried out capsid modification to generate recombinant variants with new potential.
AAV Capsid Modification
Capsid modification refers to the intentional modification of hypervariable loops (and possibly other regions) of the capsid to achieve a desired biological property. To quantify carrier results, site-specific installation of antigen-specific ligands is usually preferred. However, the scale of modifying the capsid (whether or not it disrupts its assembly) is influenced by the size of the integration sites and insertion ligands. It is also an influencing factor on the stoichiometry of individual VP in each garment shell. For example, inserting a scaffold into the unique structural domain of VP1 for affinity binding will exhibit unpredictable capsid structure and assembly. In contrast, modifying the overlapping region of VP1/VP2 with non-homologous peptides will result in a chimeric AAV vector that retains considerable transduction characteristics and higher vector genome copy numbers. Due to the fact that VP1 is the most abundant capsid protein and contains key motifs required for viral infectivity and capsid assembly, it is necessary to fully understand the insertability through computer-guided simulation studies (especially within the unique region of VP1). Another important aspect of AAV capsid modification is the type of antigen targeting portion that modifies the capsid protein.
What Are the Components of the AAV Capsid?
The AAV capsid is composed of three different viral proteins (VP1, VP2, VP3) in a ratio of 1:1:10. Manipulating one or more VPs involves introducing ligands and scaffolds to enhance tissue specificity, increase packaging yield, conceal vectors to trigger anti AAV immune responses, and so on. Capsular modification has been shown to significantly enhance the transduction activity of AAV vectors, thereby reducing anti host immune responses. Due to the interaction between AAV and host cells through its capsid protein, it remains an important component of AAV based gene delivery vectors for cell targeting and mediating immune toxicity.
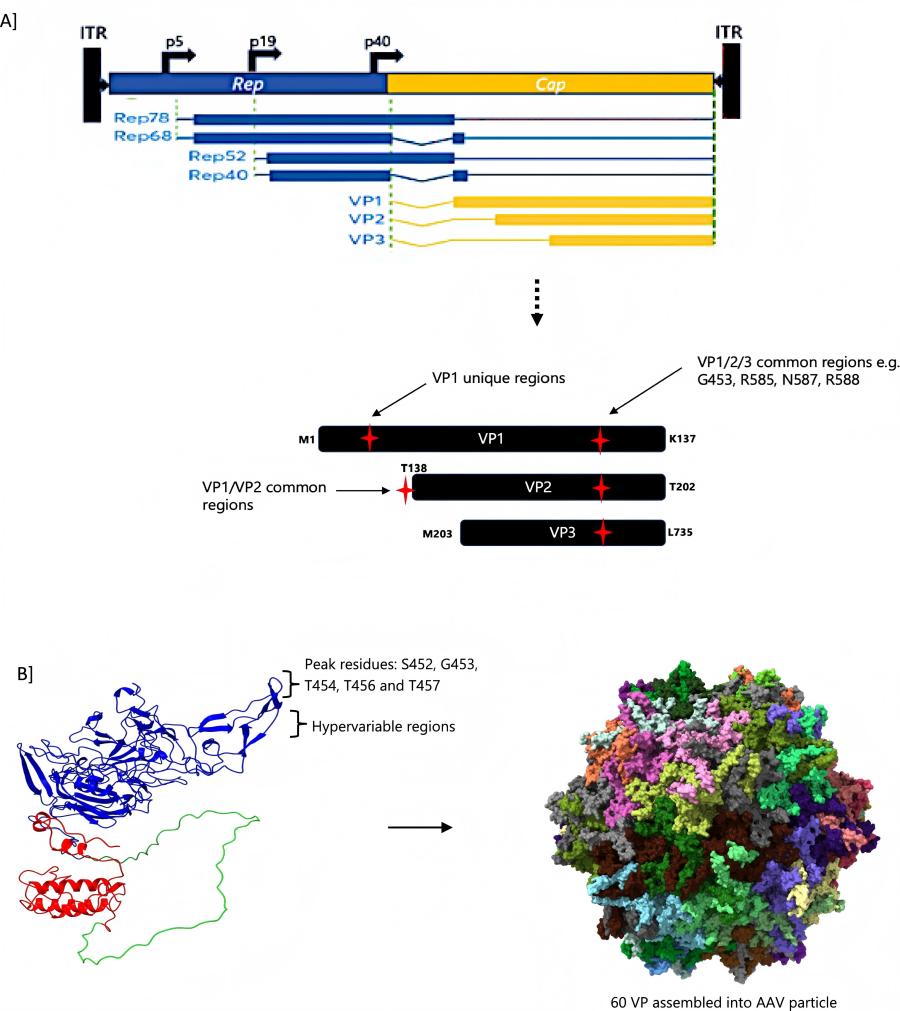 Figure 1 An illustration of AAV capsid protein common modification sites (A) and representation of capsid structure showing a monomer (VP1-red, VP2-green and VP3-blue) and the assembled 60 monomers (B) (PDB ID 5IPI).1
Figure 1 An illustration of AAV capsid protein common modification sites (A) and representation of capsid structure showing a monomer (VP1-red, VP2-green and VP3-blue) and the assembled 60 monomers (B) (PDB ID 5IPI).1
Core Services at Creative Biolabs
Creative Biolabs is at the forefront of providing comprehensive solutions for AAV capsid modification. We provide customized AAV capsid engineering services based on specific customer needs. Our experienced team of scientists utilizes state-of-the-art technologies, including advanced structural biology methods for structure guided design and high-throughput screening techniques for library selection.
- Customized AAV Capsid Engineering: Develop novel capsids suitable for your specific research objectives, including enhancing tropism, reducing immunogenicity, and improving stability.
- AAV Library Construction: Create customized high complexity AAV capsid libraries for directed evolution and high-throughput screening.
- Vector Production and Purification: Large scale, high titer production of AAV vectors for various serotypes and engineered capsids. Our services include comprehensive purification and quality control to ensure the integrity of our products.
Our Methods of AAV Capsid Modification
There are various methods for modifying AAV capsids, mainly divided into two categories: rational design and directed evolution. These methods are typically used in complementary ways to achieve a leap in AAV vector performance.
Rational Design of AAV Capsid
Reasonable design relies on a deep understanding of AAV capsid structure and function. Scientists precisely introduce mutations at specific locations on the capsid to achieve the desired effect.
- Epitope Deletion
- Targeting Peptide Insertion
- Chemical Surface Modification
Directed Evolution of AAV Capsid
Directed evolution is a high-throughput, unbiased method that simulates natural selection to screen AAV capsid variants with desired features. This method does not require prior structural information and can discover unexpected and functionally superior shells.
- Library Construction
- In Vitro/In Vivo Screening
- Sequencing and Iteration
Why Choose Creative Biolabs' Services
Our expertise in AAV capsid modification is supported by a deep understanding of virology, structural biology, and gene therapy. We leverage proprietary technology and a team of highly skilled scientists to provide key advantages.
Deep Scientific Expertise
Our team of doctoral level scientists has extensive experience in AAV biology and vector engineering, ensuring a strategic and informed approach to each project.
Cutting-Edge Technology
We use the most advanced tools, including next-generation sequencing, advanced bioinformatics, and high-throughput screening platforms, to quickly identify and characterize the best performing AAV variants.
Customization and Flexibility
We recognize that every treatment plan is unique. Our services are fully customizable to meet your specific needs, whether you require new serotypes or optimized carriers for difficult to target tissues.
Quality and Reliability
We are committed to the highest standards of quality control and product consistency, providing carriers suitable for preclinical and research purposes.
Frequently Asked Questions
Q: How do you ensure the stability of modified AAV capsids?
A: In the design phase, we use computational tools and structural analysis to predict the impact of modifications on the stability of the garment shell. After generating the modified capsid, we conducted a series of biophysical tests, such as thermal stability testing and protease digestion testing, to evaluate its stability. If necessary, we can make additional modifications or adjust the production process to improve the stability of the garment shell.
Q: Can you modify the AAV capsid to target specific cell types that cannot be well targeted by natural serotypes?
A: Yes, by combining structure-oriented design with library-based screening, we can design AAV capsids to target specific cell types. We first identify potential receptor binding regions on the capsid that can be modified to interact with receptors present on the target cell type. Then, we generate a library of capsid variants and screen them in vitro and in vivo to select variants with the desired cell type specific orientation.
Q: Can the improved AAV capsid overcome existing immunity?
A: Yes, this is a critical application. By designing capsids that are structurally different from common wild-type AAV serotypes, we can create vectors that are unlikely to be neutralized by pre-existing antibodies in patients, making gene therapy more widely accepted by the population.
Reach Out to Us Now!
Creative Biolabs' team is composed of highly skilled scientists with extensive experience in AAV research and capsid modification. Many of our researchers have been involved in pioneering research related to AAV vector development, and this expertise enables us to provide innovative solutions to our clients. We have successfully completed numerous projects for academic institutions, biotechnology companies, and pharmaceutical companies, demonstrating our ability to handle complex and diverse needs. Please feel free to contact us for more details and our scientists will conduct further in-depth discussion on your project.
Reference
- Dogbey D M, Barth S. AAV capsid modification and its influence on viral protein stoichiometry and packaging fitness: current understandings and future direction. Molecular Biotechnology, 2025: 1-9. https://doi.org/10.1007/s12033-025-01381-0 (Distributed under Open Access license CC BY 4.0, without modification.)
