Genetic Modification Services for AAV Vector
Introduction
Genetic Modification of AAV Vector optimizes AAV's capsid and genome—modifying tropism, fitting larger genes, enhancing expression, and reducing immunogenicity to solve key gene delivery hurdles. Creative Biolabs' service offers tailored solutions with advanced techniques, rigorous QC for batch consistency, GLP-compliant data for clinical use, and efficient workflows to accelerate research and de-risk development.
Discover How We Can Help - Request a Consultation
Genetic Modification of AAV Vector
The AAV vector genome is single-stranded DNA, and its modification mainly focuses on core regions such as regulatory elements, therapeutic genes, and inverted terminal repeats (ITRs) to improve expression efficiency, control spatiotemporal specificity of expression, and reduce risks.
Regulatory Element Optimization (Promoter/Enhancer Engineering)
- Replace natural promoters: Select tissue-specific promoters (e.g., liver-specific ApoA1 promoter, neuron-specific Synapsin promoter) to achieve specific expression of therapeutic genes in target tissues/cells and reduce off-target effects;
- Add enhancers/insulators: Inserting enhancers upstream of the promoter can improve transcription efficiency, while adding insulators can prevent abnormal activation of adjacent genes caused by vector integration, enhancing safety.
- Therapeutic Gene Optimization
- Codon optimization: Adjust therapeutic gene sequences per target cell codon preference, remove rare codons and unstable RNA elements to boost mRNA translation.
- Fusion tags/signal peptides: Add fluorescent tags (e.g., GFP) for vector tracking, or secretion signal peptides to enable recombinant protein secretion.
Inverted Terminal Repeats (ITRs) Modification
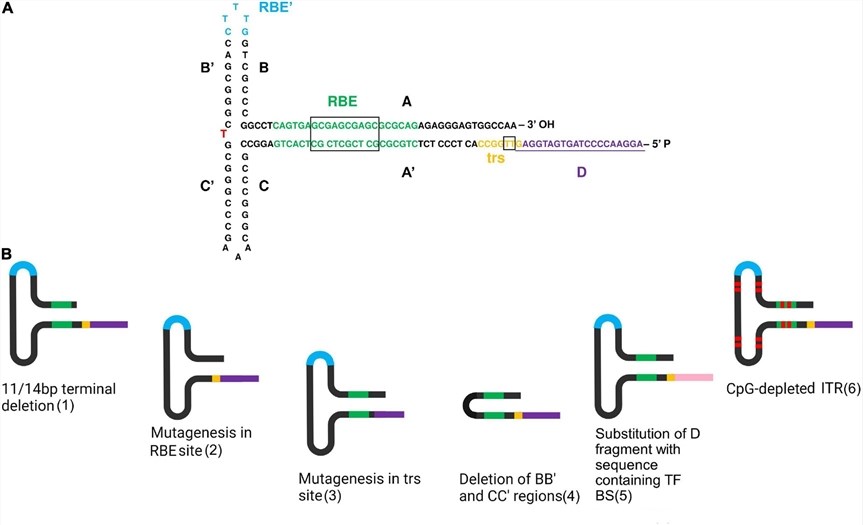 Fig.1 Directed structure modification of ITRs. Including 11/14 bp terminal deletion, RBE site mutation, trs site mutation, BB 'and CC' region deletion, D region replacement of 5 'ITR, CpG depletion modification, etc.1
Fig.1 Directed structure modification of ITRs. Including 11/14 bp terminal deletion, RBE site mutation, trs site mutation, BB 'and CC' region deletion, D region replacement of 5 'ITR, CpG depletion modification, etc.1
Mutate or modify specific ITR regions (key for replication/packaging) to regulate vector replication efficiency, expand packaging capacity, or reduce non-specific host genome integration risk.
Deletion/Replacement of Redundant Sequences
Remove non-essential backbone sequences (e.g., wild-type rep/cap genes, supplied by packaging cells) to free space for longer therapeutic genes, expanding beyond AAV's natural ~4.7kb capacity.
Introduction of Immune Regulatory Elements
Insert immune-inhibitory elements (e.g., CTLA-4Ig coding sequences) or delete pro-inflammatory sequences to reduce post-infusion immune rejection (e.g., complement activation, T cell response).
Peptide Insertion for Cell Surface Targeting of Advanced AAVs Vector
The AAV capsid dictates tissue tropism. To achieve precise targeting beyond natural serotypes, we insert receptor-specific ligands into capsid variable regions. This redirects vectors to desired sites (e.g., skeletal muscle, CNS) while avoiding off-target tissues like the liver.
Rational Design-Based Capsid Amino-acid Mutation
We use rational design to mutate specific capsid amino acids, leveraging serotype structure knowledge to alter tropism, reduce immunogenicity, and enhance barrier-crossing (e.g., blood-brain barrier). AI-driven design accelerates identifying optimal mutations, enabling custom vectors tailored to therapeutic needs.
Workflow
-
Required Starting Materials: To initiate a project, we typically require the following information and materials:
- The gene of interest (GOI) sequence and any desired regulatory elements (e.g., promoters, enhancers).
- The target cell or tissue type for gene delivery.
- Specific project goals, such as desired expression levels or therapeutic outcomes.
- Project Design & Strategy: We start with a detailed consultation to understand your needs, then design a customized vector engineering strategy—selecting optimal serotype and proposing capsid, genome, or ITR modifications to enhance performance.
- Genetic Modification & Vector Construction: Execute precise modifications based on design, including gene cassette optimization for expression, capsid engineering for tropism/immunogenicity, and Rep/ITR adjustments to boost yield and reduce empty capsids.
- Vector Production & Purification: Generate high-titer, high-purity AAV via validated platforms; advanced purification removes impurities and empty capsids, critical for preclinical/clinical safety and efficacy.
- Quality Control (QC) & Validation: Implement multi-layered QC to verify integrity, titer, and purity, using methods like ddPCR for genome quantification and TEM for particle analysis.
- In Vitro & In Vivo Testing (Optional): Offer customized in-house testing to validate modified vectors in relevant cell lines or animal models, demonstrating improved transduction and in situ expression.
-
Final Deliverables: Upon project completion, you will receive a comprehensive package that includes:
- Detailed Project Report: An in-depth report summarizing all steps of the workflow, including methods, QC data, and a full sequence map of the final vector.
- Purified AAV Vector: The final, high-quality AAV vector, ready for your downstream applications.
- Raw Data and Analysis: All raw data from QC and validation steps, ensuring complete transparency and reproducibility.
- Estimated Timeframe: The typical timeframe for this service ranges from 6 to 12 weeks, depending on the complexity of the genetic modifications and the scope of required QC and validation services.
What we can offer
Customized Vector Design
We provide a highly tailored approach, modifying AAV vectors to meet your specific project requirements, including targeted tropism and optimized gene expression for your unique application.
Holistic Vector Optimization
Our expertise extends beyond standard serotype selection to a comprehensive approach that includes rational capsid design, genomic (scAAV) engineering, and modification of Rep/ITR elements to enhance production and performance.
Advanced Capsid Engineering
We specialize in sophisticated capsid modifications, including peptide insertions and rational amino-acid mutations, to achieve precise tissue targeting and circumvent pre-existing immunities.
AI-Driven Design
We utilize cutting-edge, data-driven, and AI-guided design principles to explore novel AAV serotypes with superior properties, ensuring you have access to next-generation gene delivery technology.
End-to-End Solutions
From initial consultation and vector design to high-titer production, rigorous quality control, and optional in vitro/in vivo validation, we offer a seamless, one-stop workflow to accelerate your project.
Expert Guidance
Our team of specialists provides scientific consultation at every stage, helping you navigate the complexities of AAV vector selection and optimization to ensure the success of your gene therapy program.
Customer Reviews

FAQs
30% of animals have AAV neutralizing antibodies (Nab), reducing transduction. Can modification mitigate this? What's the recovery rate? Do you provide GLP data?
Yes, we can mitigate this issue by mutating the immunodominant epitopes of the VP3 protein, which reduces Nab binding to the vector. In mouse models, the transduction efficiency recovery rate reaches ≥60% when Nab titer is 1:10, and ≥35% when Nab titer is 1:100. We also offer GLP-compliant reports, including expression efficiency curves under different Nab titers, serum cytokine profiles, and immunogenicity comparisons with clinically used AAV variants, to support your IND applications.
The previously modified AAV had a 15% titer CV. What's your QC standard? How to ensure batch consistency?
Our QC system is tailored for modified AAV vectors: we use ddPCR for absolute titer quantification, controlling the error within ≤5%; and SEC-MALS for purity detection, ensuring the empty capsid rate is below 10%. To guarantee batch consistency, we implement a "dual-track traceability" approach—retaining original clones of capsid libraries and using single-colony amplification for genomic vectors. Transduction efficiency is tested synchronously in HEK293 cells and target cells across batches, with the CV value strictly controlled below 8%, and we provide verification data for 3 consecutive batches.
Contact Our Team for More Information and to Discuss Your Project
To learn more about our services or to initiate a project, please reach out to our team of experts. We are committed to helping you achieve your research goals with our cutting-edge AAV vector engineering solutions.
Reference
- Shitik, Ekaterina M., Igor K. Shalik, and Dmitry V. Yudkin. "AAV-based vector improvements unrelated to capsid protein modification." Frontiers in Medicine 10 (2023): 1106085. https://doi.org/10.3389/fmed.2023.1106085. Distributed under Open Access license CC BY 4.0, without modification.
