AAV Vector Design Service for CRISPR mediated In Vivo Genome Editing
Introduction
The convergence of CRISPR-Cas9 (which uses guide RNA and Cas protein for precise DNA editing) with advanced viral delivery systems ushers in a new medical era. CRISPR has great potential for genetic disease treatment, but relies on safe, efficient in vivo component delivery—AAV vectors excel here, with strong safety, low immunogenicity, and ability to transduce dividing/non-dividing cells for long-term expression.
To tackle gene therapy challenges (limited delivery, off-target effects, and non-integrating needs), our custom AAV Vectors for CRISPR In Vivo Editing use advanced engineering and high-throughput screening for a specific, safe platform. It enables stable, efficient CRISPR expression and tailors AAV vectors to needs like mutation correction or disease model development.
Discover How We Can Help - Request a Consultation
AAV Vectors for CRISPR-Mediated In Vivo Genome Editing
As a revolutionary gene-editing tool, CRISPR-Cas9 enables the highly specific and permanent modification of the genome. The system functions like a molecular scissor, using a guide RNA to direct a Cas protein to a precise location in the DNA, where it can be cut, inserted, or repaired. This capability opens doors to correcting the root cause of many diseases, from single-gene disorders like Huntington's disease to more complex conditions like Alzheimer's.
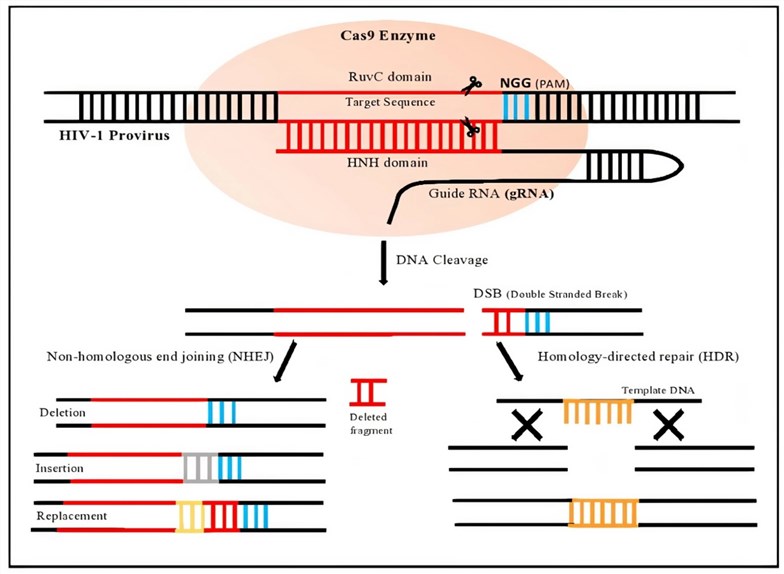 Fig.1 Schematic diagram of gene editing using CRISPR/Cas9.1,3
Fig.1 Schematic diagram of gene editing using CRISPR/Cas9.1,3
AAV and CRISPR: CRISPR's clinical application relies on robust delivery, and AAV vectors are the preferred in vivo choice. Small, non-pathogenic, and replication-defective, they efficiently carry CRISPR cargo (Cas protein, guide RNA). Selecting the right AAV serotype is key—each has unique tissue tropism (e.g., muscle, liver, CNS) for targeted delivery.
Advantages
- High tissue specificity, reducing the risk of off-target effects in unintended organs
- Non-integrating property, mitigating the risk of insertional mutagenesis
- Long-term transgene expression with a single treatment, offering curative potential
Workflow
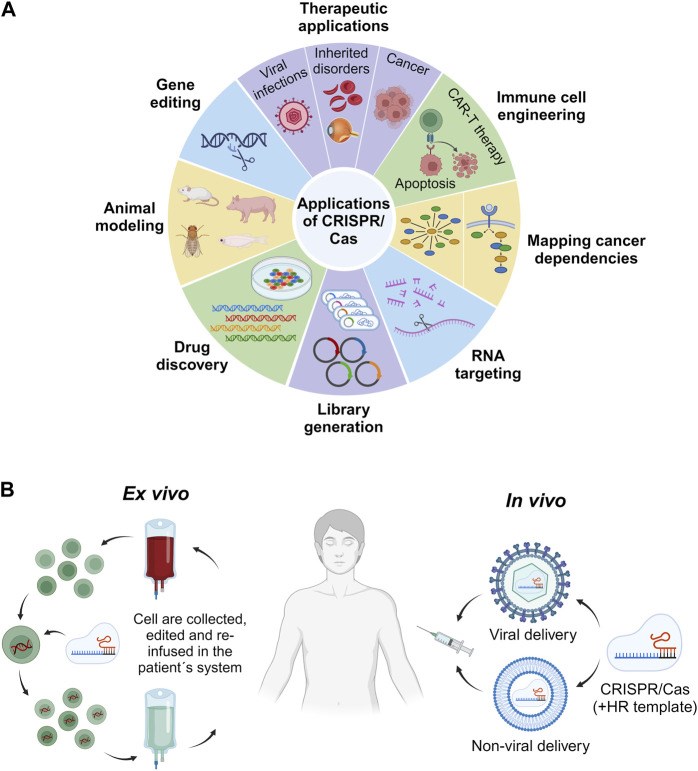 Fig.2 The application of CRISPR/Cas and its ex vitro and in vivo strategies, including cell collection, in vitro editing through CRISPR/Cas, and cell reinfusion into patients.2,3
Fig.2 The application of CRISPR/Cas and its ex vitro and in vivo strategies, including cell collection, in vitro editing through CRISPR/Cas, and cell reinfusion into patients.2,3
- Required Starting Materials: Project initiation needs characterized materials—plasmid with target gene/gRNA, target gene info (name, sequence, loci), and desired in vivo model/tissue tropism—to design effective AAV vectors.
- Vector Design and Plasmid Construction: Experts design AAV plasmids (incorporate target gene/CRISPR components, optimize packaging/expression) and select a suitable serotype based on target tissue for specificity.
- Transfection and Viral Production: Use high-yield triple-plasmid transfection to introduce vectors into host cells for recombinant AAV production, optimizing for titer and purity.
- Harvesting and Purification: Harvest viral particles from cell culture, then purify via multi-stage chromatography to remove impurities/empty capsids for high-purity vectors.
- Quality Control and Validation: Conduct rigorous QC—qPCR titration, SDS-PAGE (capsid integrity), endotoxin/sterility testing—to ensure vector safety and quality.
- Final Formulation and Delivery: Formulate purified vectors in a stable buffer, deliver to labs with a technical report including all QC data.
- Final Deliverables: Provide in vivo-suitable high-quality AAV vectors, Certificate of Analysis (CoA) with QC data, and project report summarizing workflow/findings.
- Estimated Timeframe: 4-8 weeks, depending on construct complexity, serotype, and production scale.
What we can offer
Customized Vector Design and Optimization
We provide end-to-end support, from the initial design of your AAV vector to codon optimization, ensuring your gene expression is maximized for your specific target organism.
GMP-Certified Production
Our production process adheres to stringent Good Manufacturing Practice (GMP) standards, providing you with a high-quality product suitable for clinical applications.
Robust Quality Control
We utilize advanced analytical techniques to ensure product quality, including assays for full-to-empty capsid ratio, aggregation, and host cell protein contamination, providing you with reliable and reproducible results.
End-to-End Service
We are your one-stop partner, handling all aspects from small-scale laboratory production to large-scale industrial fermentation, ensuring a smooth transition as your project progresses.
Comprehensive Documentation
Our rigorous quality assurance procedures guarantee that all documentation, from strain origin to quality control reports, is meticulously assessed and approved.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How do I choose the right AAV serotype for my project?
The choice of serotype depends entirely on your target tissue. Each serotype has a distinct tropism. For example, AAV9 is often used for CNS applications. Our experts can help you select the optimal serotype based on your project goals and a comprehensive review of the latest research.
Can your AAV vectors accommodate large genes or multiple gRNAs for multiplex editing?
We offer customized AAV vector services: For large genes, we adopt advanced strategies, such as splitting AAV, to bypass the packaging limit of ~5kb; For multiple editing, we designed a carrier to carry multiple gRNAs. In addition, we also offer tailor-made single-gene and multi-gene delivery solutions to fully meet your research needs.
Are your services suitable for clinical applications?
Our services are primarily tailored for preclinical research. However, the high-quality standards and robust quality control we employ are designed to facilitate a smooth transition to GMP manufacturing. We can provide consultation on the requirements for clinical-grade vectors and connect you with partners for scale-up.
How does your service compare to using other delivery methods like lipid nanoparticles (LNPs)?
While LNPs have shown promise, especially for liver-targeted applications, AAV vectors offer superior sustained expression and have demonstrated a broader tropism for a variety of tissues. For long-term, tissue-specific, and non-integrating gene delivery, AAV remains the gold standard. We can discuss the pros and cons of each method to help you make an informed decision for your project.
We are dedicated to helping you achieve your research and therapeutic goals. Our team of specialists is ready to discuss your specific needs and provide a customized solution to accelerate your project. Reach out to us today to learn how our advanced AAV vector services can empower your next breakthrough.
Contact Our Team for More Information and to Discuss Your Project
References
- Bhowmik, Ruchira, and Binay Chaubey. "CRISPR/Cas9: a tool to eradicate HIV-1." AIDS Research and Therapy 19.1 (2022): 58. https://doi.org/10.1186/s12981-022-00483-y.
- Macarrón Palacios, Arturo, et al. "Revolutionizing in vivo therapy with CRISPR/Cas genome editing: breakthroughs, opportunities and challenges." Frontiers in Genome Editing 6 (2024): 1342193. https://doi.org/10.3389/fgeed.2024.134219.
- Distributed under Open Access license CC BY 4.0, without modification.
