AAV Vector Design Service for Large Gene Delivery
Introduction
AAV vectors have revolutionized gene therapy with safe, efficient therapeutic gene delivery, but their ~5 kb capsid packaging limit hinders treating large-gene-related diseases like Duchenne muscular dystrophy. To address the challenge, our custom AAV design service uses advanced split and oversized vector strategies. Creative Biolabs' Large Gene Delivery service, built on validated split vector technologies (homologous recombination, splicing, etc.), provides expertly engineered AAV vectors for robust, long-term full-length gene expression, enabling broader genetic disease treatment and accelerating gene therapy projects.
Discover How We Can Help - Request a Consultation
Large Gene Delivery
What is a Large Gene?
A "large gene" in the context of AAV gene therapy refers to any gene whose coding sequence, along with necessary regulatory elements like promoters and polyadenylation signals, exceeds the AAV packaging capacity. A prominent example is the full-length dystrophin gene, which is over 11 kb long and a key target for Duchenne muscular dystrophy treatments.
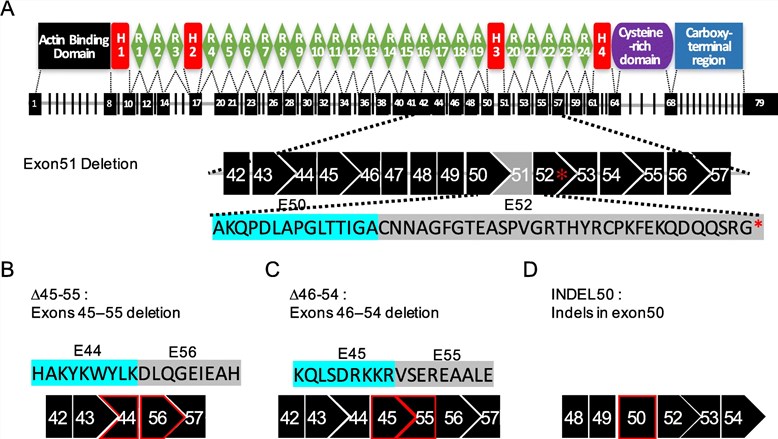 Fig.1 The structure of human dystrophin and the targeting strategies for mutant human DMD genes.1
Fig.1 The structure of human dystrophin and the targeting strategies for mutant human DMD genes.1
- Oversized AAV Vectors: Packages genes slightly exceeding the ~5 kb limit. Oversized genomes are truncated/fragmented during production, then reconstituted into functional cassettes in target cells—though with lower efficiency and higher variability.
- Dual AAV Vectors: The most widely used solution; splits large genes into two parts, each in a separate AAV. Co-delivered to target cells, where the two halves reunite via various mechanisms to express full-length protein.
- Hybrid Dual AAV Vectors: Combines multiple mechanisms (e.g., homologous recombination and splicing) to boost reconstitution efficiency and fidelity, optimizing therapeutic outcomes.
Advantages
- Expanded Cargo Capacity: It directly addresses the primary limitation of AAV vectors, allowing for the delivery of genes well over the 5 kb limit.
- Increased Versatility: It provides a platform to deliver a wide array of large therapeutic genes for diseases previously untreatable with AAV.
- Clinical Relevance: The approach has shown promising results in preclinical and clinical trials, demonstrating its potential for widespread therapeutic application.
Workflow
- Required Starting Materials: Project initiation requires clients to provide full-length therapeutic gene sequence, target cell/tissue, and desired regulatory elements (e.g., promoters).
- Project Assessment and Vector Design: Team reviews materials, analyzes gene/regulatory elements, designs custom vector strategies (e.g., dual AAV), and identifies optimal split sites.
- Gene Synthesis and Cloning: Synthesizes therapeutic gene and clones it into proprietary AAV backbones; may create multiple plasmids for dual/hybrid systems.
- Vector Production and Purification: Produces high-titer vectors via advanced cell culture, then purifies to obtain pure, functional AAV particles.
- Quality Control and Validation: Conducts extensive QC (titer, purity, integrity) and in vitro assays to validate reconstituted gene functionality and full-length protein expression.
- Final Delivery and Reporting: Ships AAV vectors with comprehensive docs (QC reports, sequence data, storage/use protocols).
- Final Deliverables: Clients receive high-titer AAV vectors, project summary report (design/validation), and raw QC data files.
- Estimated Timeframe: 8–14 weeks, depending on gene complexity and vector strategy.
What we can offer
End-to-End Custom Gene Delivery Service
A custom, end-to-end gene delivery service, from the initial consultation to the final delivery of functionally validated vectors.
Advanced Vector Engineering for Large Genes
Advanced vector engineering solutions, including dual AAV, oversized AAV, and hybrid systems, to successfully package genes up to ~11 kb in size.
Expertise in Gene Reconstitution Mechanisms
Expertise in various reconstitution mechanisms, such as trans-splicing and homologous recombination, ensuring accurate and efficient full-length gene expression.
QbD & PAT-Driven Process Development
Rigorous upstream and downstream process development with industry-leading quality-by-design (QbD) and process analytical techniques (PAT) to guarantee vector purity and high titer.
Rigorous QC & Functional Validation Workflow
A well-established quality control and validation workflow that confirms the functional expression of the therapeutic protein in vitro, giving you confidence for your in vivo or ex vivo applications.
Tailored Collaborative Research Support
Tailored and collaborative services that can be adjusted to your specific research requirements, from gene optimization to a fully customized vector design.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How does the dual AAV approach ensure both vectors enter the same cell?
While co-transduction efficiency can vary, our vector designs and administration protocols are optimized to increase the likelihood of both vectors entering the same target cell. Our expert team can guide on administration routes and doses to maximize co-transduction and gene expression.
What is the risk of the two gene halves not re-joining correctly?
We use robust reconstitution mechanisms, such as trans-splicing and homologous recombination, which are designed to ensure accurate reassembly. Our quality control process includes assays that specifically confirm the production of the full-length, functional protein, mitigating the risk of incorrect joining.
Are there any alternatives to the dual AAV strategy for large genes?
Yes, in some cases, an oversized AAV vector strategy can be used, although its efficiency may be lower. Our team can also help you explore other viral vectors like lentivirus, which have a larger packaging capacity, to determine the best approach for your specific project.
To learn more about how our custom AAV design and large gene delivery services can accelerate your research, please reach out to our team of experts. We are ready to answer your questions and help you design a project that meets your specific needs.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Chen, Menglong, et al. "In vivo genome editing in mouse restores dystrophin expression in Duchenne muscular dystrophy patient muscle fibers." Genome Medicine 13.1 (2021): 57. https://doi.org/10.1186/s13073-021-00876-0. Distributed under Open Access license CC BY 4.0, without modification.
