AAV Vector Design Service for Multiple Gene Delivery
Introduction
Gene therapy holds promise for genetic disorders via functional gene delivery, but AAV vectors' limited cargo capacity restricts it to single-gene diseases, leaving polygenic/complex ones without solutions. Recent advances (biological engineering, machine learning) aid multi-gene strategies. Our custom AAV Design for Multiple Genes Delivery service uses advanced engineering and data-driven methods to bypass limits, offering tailored multi-gene co-expression, precise 2-3 gene delivery, and end-to-end support from design to delivery.
Multiple Genes Delivery
When the combined size of the genes you need to deliver exceeds the typical 5 kb packaging capacity of a single AAV vector, a co-delivery system is the optimal solution.
Co-delivery of Two AAV Vectors System
For two genes too large for one vector—produce two separate AAVs (each with one gene cassette), co-administer to target cells for both genes' delivery and expression. Bypasses size limits reliably.
Co-delivery of Three AAV Vectors System
For two genes too large for one vector—produce two separate AAVs (each with one gene cassette), co-administer to target cells for both genes' delivery and expression. Bypasses size limits reliably.
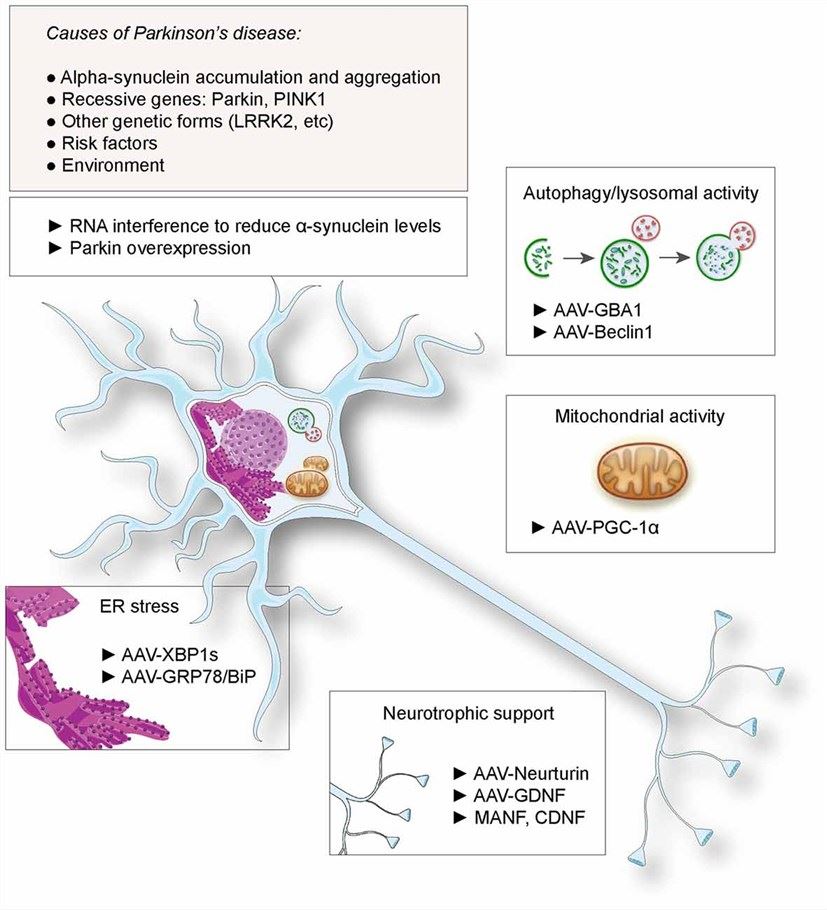 Fig.1 Neuroprotective gene therapy for Parkinson's disease (PD) and the application of AAV.1
Fig.1 Neuroprotective gene therapy for Parkinson's disease (PD) and the application of AAV.1
Advantages of Co-delivery
The primary advantage of a co-delivery system is its ability to enable the treatment of complex, polygenic disorders that are otherwise impossible to address with single-vector approaches. This expands the therapeutic possibilities of AAV gene therapy, making it a viable option for a broader range of diseases.
Workflow
- Required Starting Materials:
- Target Gene Sequences: The DNA sequences for the therapeutic genes you wish to co-express.
- Project Specifications: Detailed information on the disease model, target cell types, desired expression levels, and any specific promoters or enhancers you wish to utilize.
- Safety & Regulatory Considerations: Any known safety concerns or regulatory requirements relevant to your therapeutic target.
- Initial Consultation & Project Design: Discuss project goals/requirements, then design a custom vector strategy—select optimal AAV serotype and delivery platform to boost efficacy.
- Gene Synthesis & Vector Construction: Synthesize target genes, clone into AAV plasmids, and conduct strict QC to ensure genetic construct integrity.
- AAV Production & Purification: Produce high-titer AAV via advanced triple-plasmid transfection, then purify through multi-step processes for clinical-grade products.
- Quality Control & Functional Validation: Run comprehensive QC (titer, purity) and functional assays to confirm vector potency and safety.
- Final Delivery: Provide purified, validated AAV vectors plus a detailed report with relevant data and protocols.
- Final Deliverables:
- Final AAV Vector: A stock of the purified and ready-to-use AAV vector.
- Comprehensive QC Report: Detailed results from all quality control assays (e.g., SDS-PAGE, DLS, qPCR).
- Detailed Experimental Protocol: A complete protocol outlining the vector design, production, and purification steps.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 12 weeks, depending on the complexity of the gene sequences and the specific AAV delivery system required.
Discover How We Can Help - Request a Consultation
What We Can Offer
Creative Biolabs offers an unparalleled, one-stop solution for your gene delivery needs, from the initial lab-scale design to large-scale, clinical-grade production. Our comprehensive services are meticulously engineered to provide you with the highest quality and most reliable AAV vectors.
One-stop service from laboratory scale, pilot scale, to large scale, ensuring a seamless transition as your project scales up.
Efficient upstream and downstream process development that is optimized to maximize the yield and quality of your AAV vectors.
Scalable industrial production with flexible processes, including batch, fed-batch, or continuous modes, to meet the specific demands of your project and maximize yield.
Customized services, including the optimization of codon usage for your genes to facilitate superior expression in a wide range of host cells.
Well-established quality system, adhering to the principles of Quality-by-Design (QbD) and process analytical techniques (PAT) to guarantee consistency and safety.
Strict aseptic verification procedures throughout our production process and adhere to Good Manufacturing Practice (GMP) principles.
High-standard quality control tools are used to quantify and evaluate the quality of every vector, from titer measurement to functional validation, ensuring you receive a product you can trust.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How do I know which delivery system is right for my project?
The best system depends on the size of your genes and the number of genes you need to deliver. We recommend starting with a consultation to discuss your specific needs. Our team will analyze your gene sequences and project goals to recommend the most efficient and cost-effective approach.
Is the co-delivery of multiple AAV vectors efficient? Do all vectors get into the same cell?
Yes, our co-delivery platforms are designed for high efficiency. We utilize optimized AAV serotypes and high-titer production to maximize the chances of co-transduction, ensuring that the target cells receive all necessary vectors and express all therapeutic genes. Our quality control processes validate this co-expression to give you confidence in your results.
Are there any safety concerns with using multiple AAV vectors?
AAV vectors are known for their excellent safety profile and low immunogenicity. While the use of multiple vectors may increase the total viral load, our processes are optimized to produce highly pure vectors, minimizing any potential for adverse immune responses. We also focus on providing vectors that can be administered at lower doses.
How does your approach compare to other multi-gene delivery methods?
Our custom AAV design service combines traditional expertise with cutting-edge technology, including a machine learning-driven approach to optimize vector capsids for multiple desirable traits. This allows us to deliver a superior product with enhanced efficacy and manufacturability, distinguishing us from less-specialized providers.
Can you help with the design of my therapeutic gene constructs in addition to vector production?
Absolutely. Our team of experts can assist with every step of the process, including gene construct design, codon optimization, and selecting the most appropriate promoters and regulatory elements for your specific application. Our service is a comprehensive solution, not just a production line.
Our dedicated team is ready to assist you in designing and executing your next-generation gene therapy project. With our deep scientific expertise and state-of-the-art AAV vector platforms, we are confident we can help you achieve your research and therapeutic goals.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Valdés, Pamela, and Bernard L. Schneider. "Gene therapy: A promising approach for neuroprotection in Parkinson's disease?." Frontiers in neuroanatomy 10 (2016): 123. https://doi.org/10.3389/fnana.2016.00123. Distributed under Open Access license CC BY 4.0, without modification.
