LNP based Antisense Oligonucleotide (ASO) Encapsulation Service
Introduction
Lipid nanoparticles (LNPs) are one of the important technologies in lipid delivery systems. Antisense oligonucleotides (ASOs) are encapsulated in LNPs to resist nuclease degradation and improve cellular delivery. Creative Biolabs has extensive experience in LNP encapsulation of ASO and can provide customers with custom LNP formulation solutions for ASOs and high-quality encapsulated products.
Overview
LNPs have a micellar structure within the particle core and can form a stable dispersed structure. ASOs encapsulated in LNPs are protected during delivery, released in cells, and translated into therapeutic proteins. A typical LNP contains 4 lipid components: ionizable lipids, PEGylated lipids, phospholipids, and cholesterol.
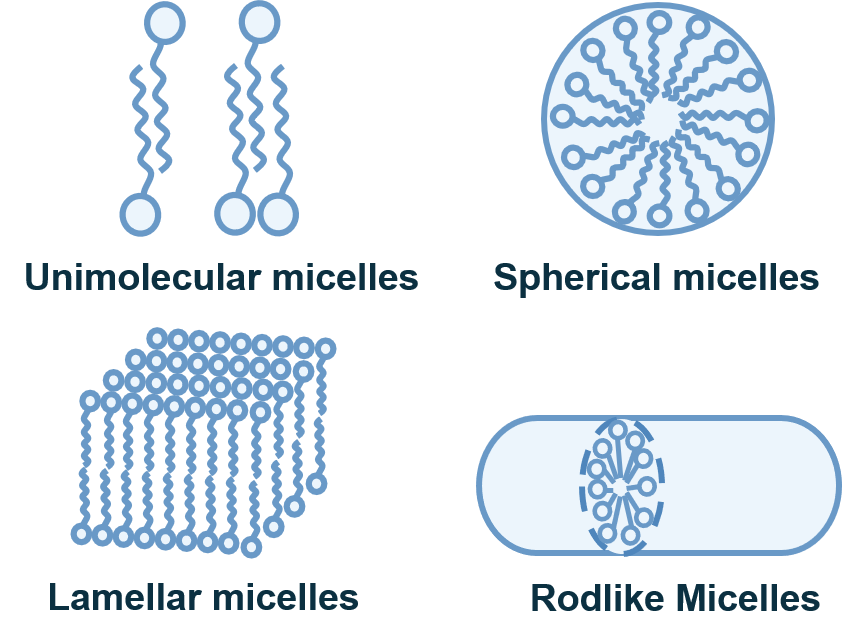 Fig.1 Micellar structure.
Fig.1 Micellar structure.
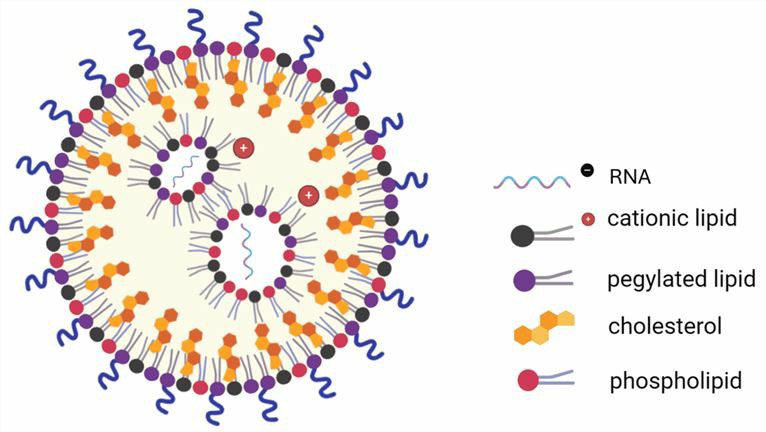 Fig.2 A typical LNP structure.1
Fig.2 A typical LNP structure.1
Lonizable lipid and cationic lipid:
Ionized lipids and cationic lipids are composed of an amino head, a hydrophobic tail, and a linker, and can be divided into monoamines and polyamines according to the number of amino heads. There are three ionizable cationic lipids approved for RNA delivery: DLin-MC3-DMA (MC3), SM-102, and ALC-0315.
- Head group
The head group is positively charged and can coat the negatively charged ASO while stabilizing the LNP to interact with the cell membrane and promote cellular uptake. Typical head groups include amines (primary amine, secondary amine, tertiary amine, quaternary amine), guanidine, heterocyclic groups, etc. During formation, the ionizable lipid and cationic lipid are positively charged by protonation and then bind to the negatively charged ASO via electrostatic interactions, thus encapsulating the ASO within the LNP.
- Linker
The linker is responsible for connecting the head and tail of the LNP, which will affect the stability, biodegradability, cytotoxicity, and transfection efficiency of the LNP. The linked fragments can be classified as either nonbiodegradable (e.g., ethers and carbamates) or biodegradable (e.g., esters, amides, and thiols).
- Tail
The tail can be a saturated/unsaturated aliphatic chain, and the degree of unsaturation affects the delivery of nucleic acids. The hydrophobic tail affects the formation and potency of LNP by affecting pKa, lipophilicity, mobility, and fusibility.
Helper lipid:
- Phospholipid: Phospholipids are accessory lipids that contribute to lipid nanoparticle formation and endosomal escape. DSPC and DOPE are the most commonly used phospholipids. To enhance the physical and biological functions of phospholipids, the synthetic pore size (SAR) of phospholipids should be considered and the chemical structure of phospholipids should be optimized.
- Cholesterol: Cholesterol helps to increase the stability of LNPs and contributes to membrane fusion.
- PEGylated lipid: PEGylated lipids can reduce nanoparticle aggregation, prolong circulation time, and escape phagocytosis by the mononuclear phagocyte system. PEG2000 lipid is most suitable for the formation of stable LNPs. Poly (glycerol), poly (oxazoline), and poly (amino acids) can be used as PEG alternatives.
The basic information of some LNP-encapsulated oligonucleotide drugs that have been approved by the FDA is shown in the table:
Table.1 Lipid composition of FDA-approved LNP drugs.
| Patisiran | BNT162b2 | mRNA-1273 | |
|---|---|---|---|
| Oligonucleotides | siRNA | mRNA | mRNA |
| Ionizable Cationic Lipid | DLin-MC3-DMA | ALC-0315 | SM-102 |
| Neutral Phospholipid | 1,2-DSPC | 1,2-DSPC | 1,2-DSPC |
| Sterol Lipids | Cholesterol | Cholesterol | Cholesterol |
| PEGylated Lipids | DMG-PEG (2000) | ALC-0159 | DMG-PEG (2000) |
| The molar ratio of lipid | 50:10:38.5:1.5 | 46.3:9.4:42.7:1.6 | 50:10:38.5:1.5 |
Synthetic method
- Thin Film Hydration Method
The lipids dissolved in organic solvents were spin-steamed to form a thin lipid layer. When the thin layer was hydrated with an aqueous buffer solution containing nucleic acids, it formed heterogeneous, nanoscale multi-layered vesicles (MLVS), which were further extruded and sonicated.
- Ethanol injection technique
The lipids were dissolved in ethanol and vesicles were formed by rapid injection under agitation into an aqueous buffer containing the drug or compound used for encapsulation.
- Microfluidics
The lipid is mixed with the ASO using a syringe pump connected to the microfluidic mixing device.
- Nanoprecipitation method
The spontaneous formation of LNP was promoted by continuous mixing of organic and aqueous phases under moderate magnetic stirring. After particle formation, the organic solvent was removed using dialysis, ultracentrifugation, rotary steaming, and freeze-drying.
- Other methods
Reverse phase evaporation method, freeze-drying method, double emulsion method, and active drug loading method (e.g., pH. gradient method, ammonium sulfate gradient method, and calcium acetate gradient method).
Quality Control
After encapsulation of ASO, the particle size, polydispersity, ζ potential, and encapsulation efficiency of the product will be examined, and the detection methods are shown in the table:
Tab.2 Quality characterization of LNP encapsulated ASO
| Particle size | Direct measurement | Transmission electron microscope (TEM) |
| Indirect measurements | Dynamic light scattering | |
| Photon correlation spectroscopy (PCS) | ||
| Polydispersity index (PDI) | TEM, Spectrophotometer, Particle size analyzer | |
| Encapsulation efficiency | Ribonuclease protection assay | |
| Absorbance-based method | ||
| Membrane-impermeable fluorescent dye exclusion assay | ||
| Density gradient ultracentrifugation | ||
| Capillary electrophoresis | ||
| ζ potential | Electrophoresis methods | |
| Electro-osmosis methods | ||
| Toluene nitrosulfonic acid (TNS) assay | ||
| Streaming current measurement | ||
Applications
Selective organ targeting (SORT)
SORT lipid nanoparticles (LNPs) deliver drugs to the liver, lung, and spleen of mice. The regulation of tissue tropism depends on the specific chemical functional groups, the physicochemical properties of SORT molecules, and the amount of SORT molecules added. In SORT, the addition of a permanent cationic lipid resulted in protein expression primarily in the lung, a permanent anionic lipid facilitated delivery to the spleen, and an ionized amino lipid increased delivery to the liver.
Advantages
Efficient encapsulation
LNP binds to negatively charged ASO through electrostatic adsorption to form a stable complex that effectively encapsulates ASO, ensuring the stability and integrity of drug delivery.
High delivery capacity
LNP with a diameter of about 100 nm has a strong ability to penetrate the cell barrier, which can deliver ASO to target cells. Moreover, LNPs can also achieve targeted delivery to specific cells by modification with specific ligands (such as GalNAc, peptides, antibodies, etc.).
Creative Biolabs offers professional services in LNP-based ASO encapsulation and assists customers in developing encapsulation strategies, high-quality production, and stringent quality control testing. If you are interested in LNP-based ASO encapsulation service, please feel free to contact us, we will be glad to serve you.
Reference
- Dinh, Linh, Lanesa Mahon, and Bingfang Yan. "Nano-Encapsulation and Conjugation Applied in the Development of Lipid Nanoparticles Delivering Nucleic Acid Materials to Enable Gene Therapies." Applied Nano 5.3 (2024): 143-161.Distributed under Open Access license CC BY 4.0, without modification.
