Polymer based Antisense Oligonucleotide (ASO) Encapsulation Service
Introduction
Polymer encapsulated antisense oligonucleotides (ASO) use polymeric materials to encapsulate ASO to improve their stability, bioavailability, and targeting. Polymers are characterized by diverse structures, simple functionalization, and excellent stability. Creative Biolabs has extensive experience in polymer encapsulation of ASO and can provide clients with custom solutions for polymer-based delivery of ASO drugs.
Overview
Polymeric materials have a variety of structures, such as linear, branched, or cross-linked structures, which can be further subdivided into star, comb, and brush shapes, etc. Polymers used to encapsulate ASO are generally biocompatible and biodegradable. The surface of polymeric materials can also be enhanced by adding targeting ligands, antibodies, nucleic acids, etc. In addition to the influence of structure, molecular weight, hydrophilicity, and charge density may also influence the functionality of polymers.
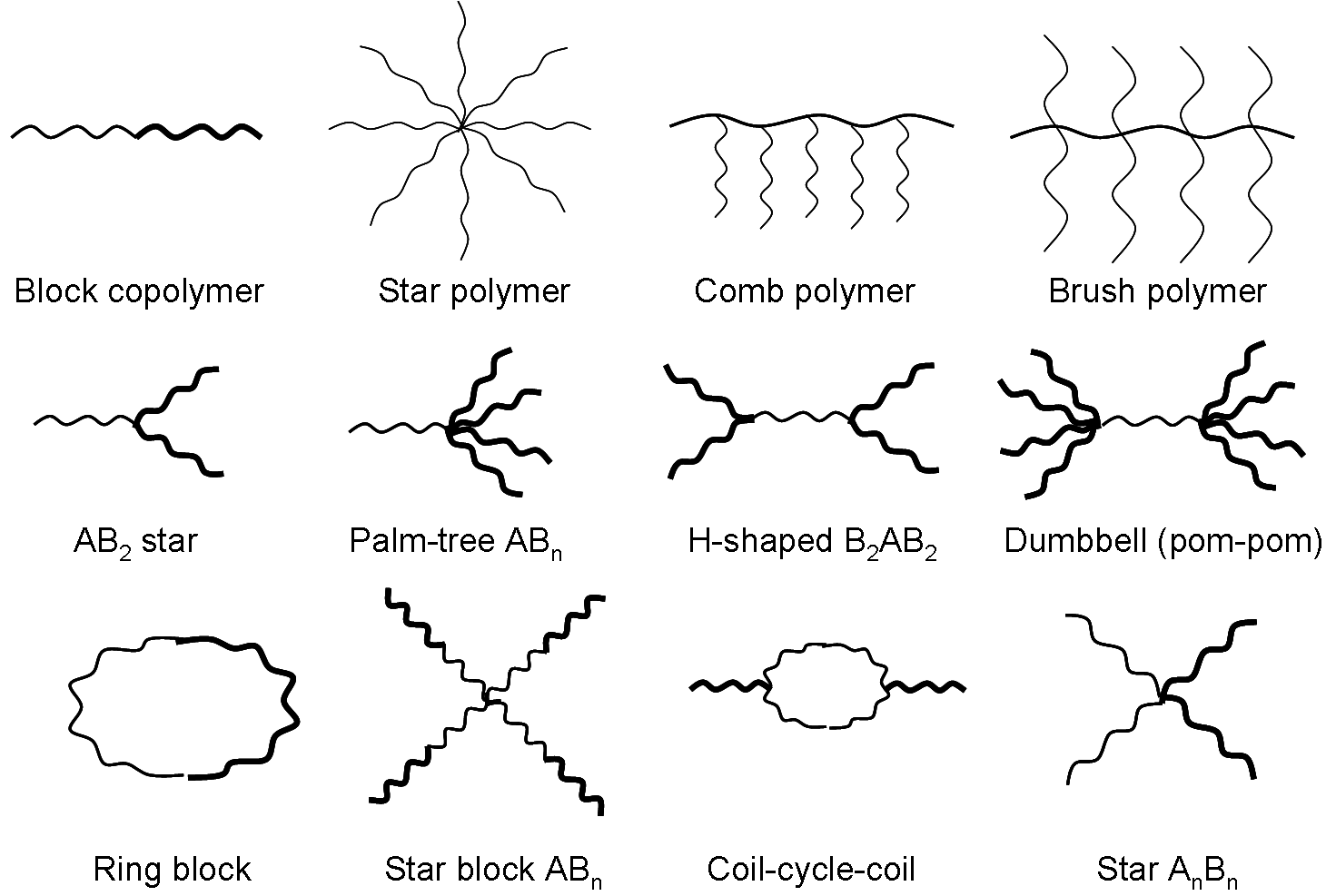 Fig.1 Different structures of polymers.Distributed under public domain, from Wiki, without modification
Fig.1 Different structures of polymers.Distributed under public domain, from Wiki, without modification
Synthesis
Tab.1 Polymer preparation methods
| Bulk polymerization | Solution polymerization | Suspension polymerization | RAFT* polymerization | |
|---|---|---|---|---|
| Component | Monomer, initiator | Monomer, initiator, solvent | Monomer, initiator, water, dispersing agent | RAFT reagent, monomer |
| Mechanism | Follow the general mechanism of free radical polymerization | Chain transfer reaction to solvent | Follow the general mechanism of free radical polymerization | The dithioester derivatives act as chain transfer reagents to form dormant intermediates with the growing chain and control the polymerization reaction. |
| Advantage | High product purity, Simple post-processing | The product is present in the solution |
Fast polymerization rate, low cost |
Mild reaction conditions, wide selection of monomers, strong molecular design capabilities |
| Disadvantage | The heat of the reaction is not easily removed |
Low polymerization rate, environmental pollution |
Dispersants are difficult to remove | Increased toxicity of polymers |
* RAFT: Reversible addition-fragmentation chain transfer polymerization.
Applications
- Dendritic macromolecules
Dendritic macromolecules have the properties of being highly branched, symmetrical, and radiating, so that the active functional groups outside the dendritic macromolecules can be conjugated with biomolecules or contrast agents to the surface, and drugs can also be loaded inside. Therefore, dendrimers can load multiple types and large quantities of drugs, improving the solubility of poorly soluble drugs. The most common research is the delivery of nucleic acids and small molecules.
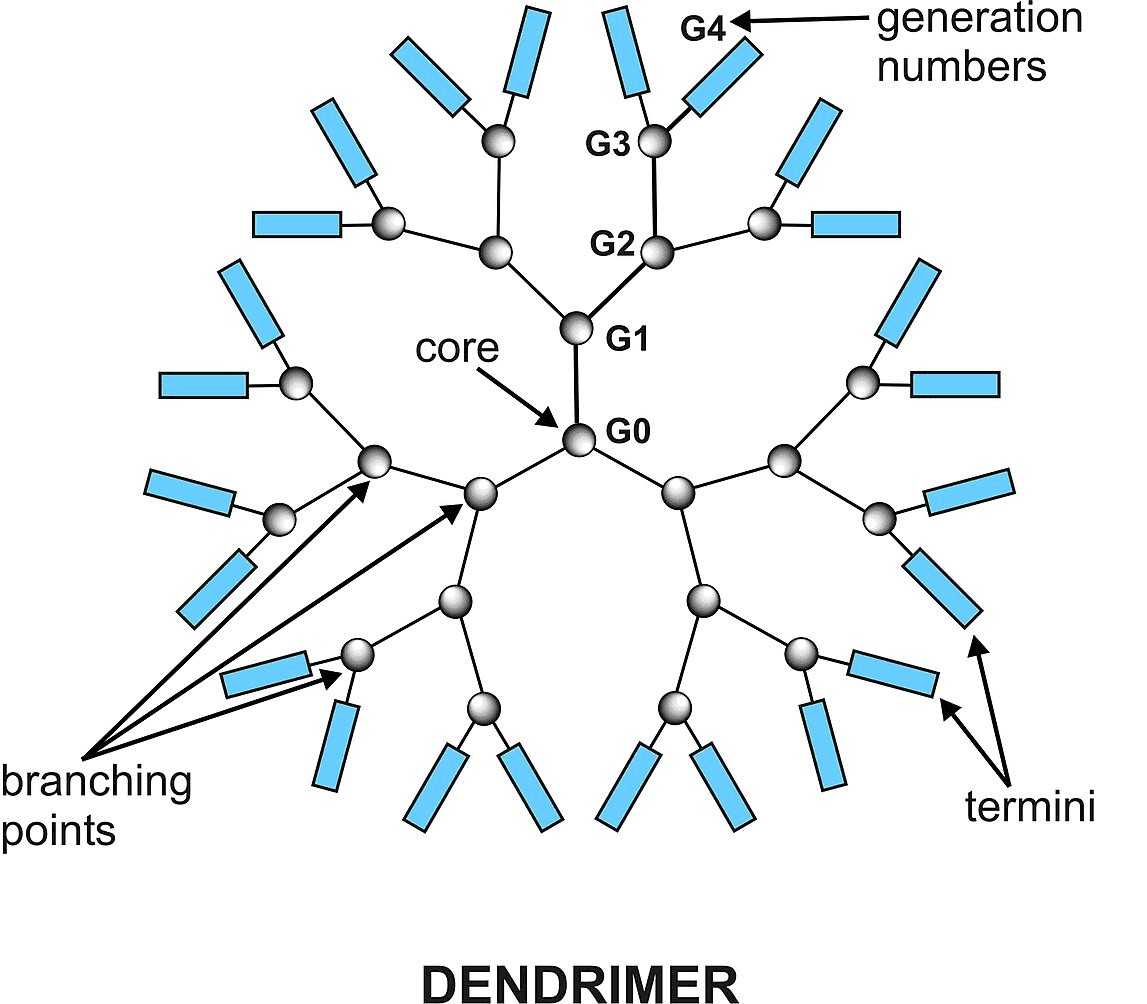 Fig.2 DendrimerDistributed under public domain, from Wiki, using only part Dendrimer of the original image.
Fig.2 DendrimerDistributed under public domain, from Wiki, using only part Dendrimer of the original image.
- Polymer nanoparticles (PNP)
PNPs are nano-sized particles made of polymer materials, which are usually between 1 and 100 nm in size and include various forms such as spherical, rod-shaped, linear, tubular, and layered. PNPs are particles or particulate materials that have a size in one dimension that is at least in the range of 10-100 nm. PNPs provide ASO drugs with better pharmacokinetic profiles and bioavailability, reduced drug toxicity, and altered drug distribution.
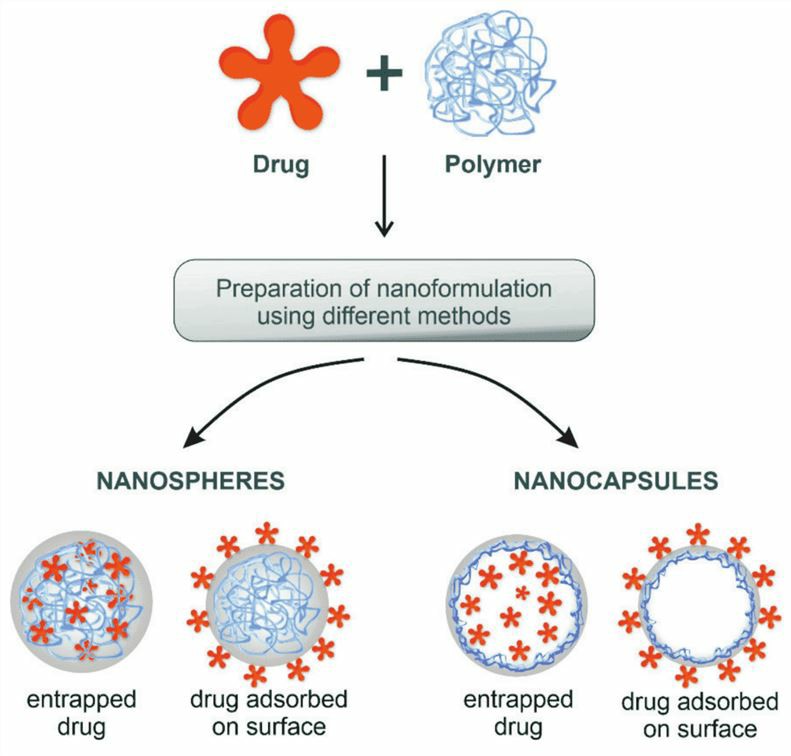 Fig.3 The polymeric nanoparticles loaded the drug.1
Fig.3 The polymeric nanoparticles loaded the drug.1
- Polyetherimide (PEI)
PEI is a water-soluble polycation composed of repetitive ethylenimine structural units, which contain a large number of amino groups and have a high buffering capacity; therefore, PEI has a strong agglomeration effect on ASO. High toxicity and low transfection efficiency limit the application of PEI. Hydrophobic modification is an effective means of improving transfection efficiency and reducing PEI toxicity.
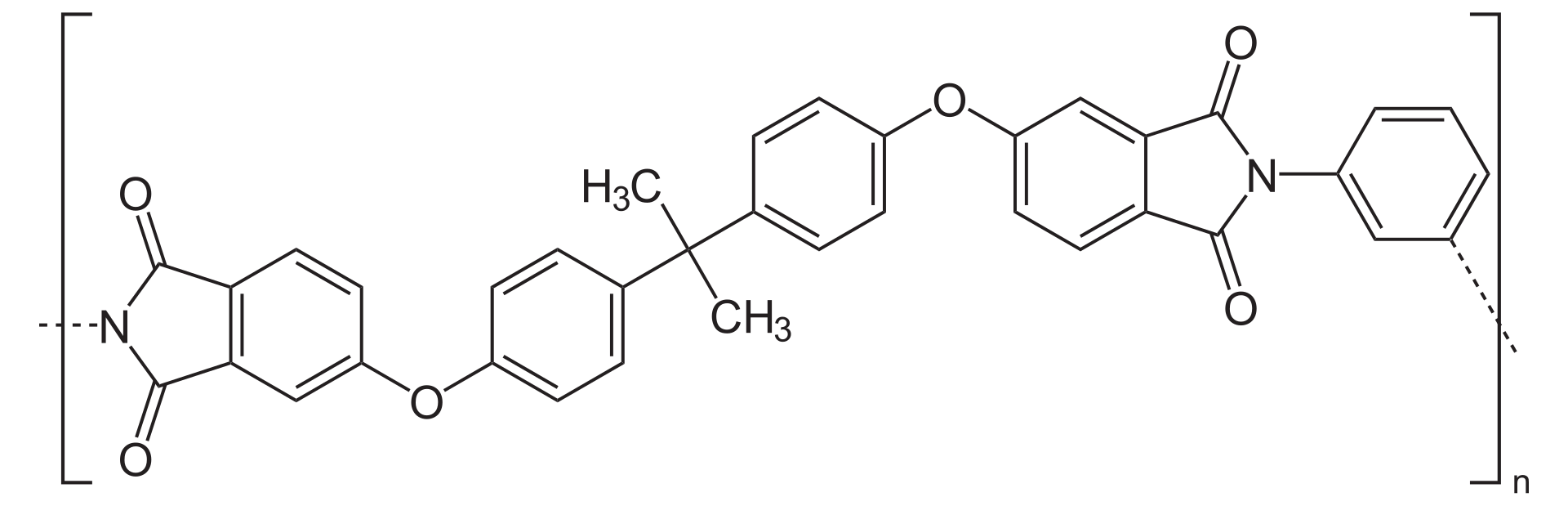 Fig.4 The structural formula of PEI.Distributed under public domain, from Wiki, without modification
Fig.4 The structural formula of PEI.Distributed under public domain, from Wiki, without modification
- Poly(lactic-co-glycolic acid) (PLGA)
The basic principle of PLGA microspheres drug loading technology is to use PLGA material to prepare small particles, and by certain methods to package or adsorb the drug inside or on the surface of the microspheres, to achieve the stable encapsulation of the drug, and by adjusting the preparation conditions and parameters of the microspheres, it is the controlled release of the drug in vivo.
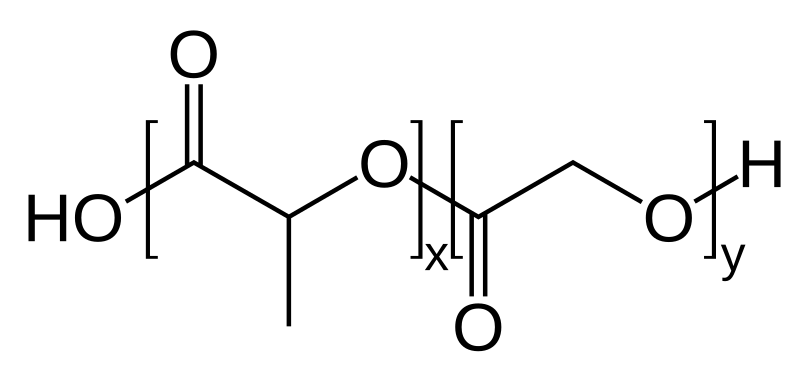 Fig.5 The structural formula of PLGA.Distributed under public domain, from Wiki, without modification
Fig.5 The structural formula of PLGA.Distributed under public domain, from Wiki, without modification
Advantages
- Controlled release:
The controlled release of ASO in vivo can be achieved by adjusting the preparation conditions and parameters of the microspheres.
- Enhanced stability
Prevent ASO from deactivating in vivo nuclease and pH environmental conditions, and prolong the action time of drugs.
- Targeted delivery
Active targeted ASO delivery through physical and chemical interactions and ligand-receptor targeting.
Creative Biolabs offers professional services in polymer-based ASO encapsulation and assists customers in developing encapsulation strategies, high-quality production, and stringent quality control testing. If you are interested in polymer-based ASO encapsulation service, please feel free to contact us, and we will be glad to serve you.
Reference
- Madej, Marcel, Natalia Kurowska, and Barbara Strzalka-Mrozik. "Polymeric nanoparticles—Tools in a drug delivery system in selected cancer therapies." Applied Sciences 12.19 (2022): 9479. Distributed Under Open Access license CC BY 4.0, without modification.
