AAV Vector Design Service for Cystic Fibrosis (CF)
Introduction
Cystic fibrosis gene therapy research faces challenges like short-lived therapeutic effects, complex drug delivery and the need for targeted approaches. Creative Biolabs' custom rAAV design service addresses these via innovative platforms and advanced molecular engineering, offering end-to-end support from early research to preclinical studies, and delivering specific, efficient vectors for precise, scalable delivery to drive drug discovery.
Discover How We Can Help - Request a Consultation
Cystic Fibrosis (CF)
Pathogenesis
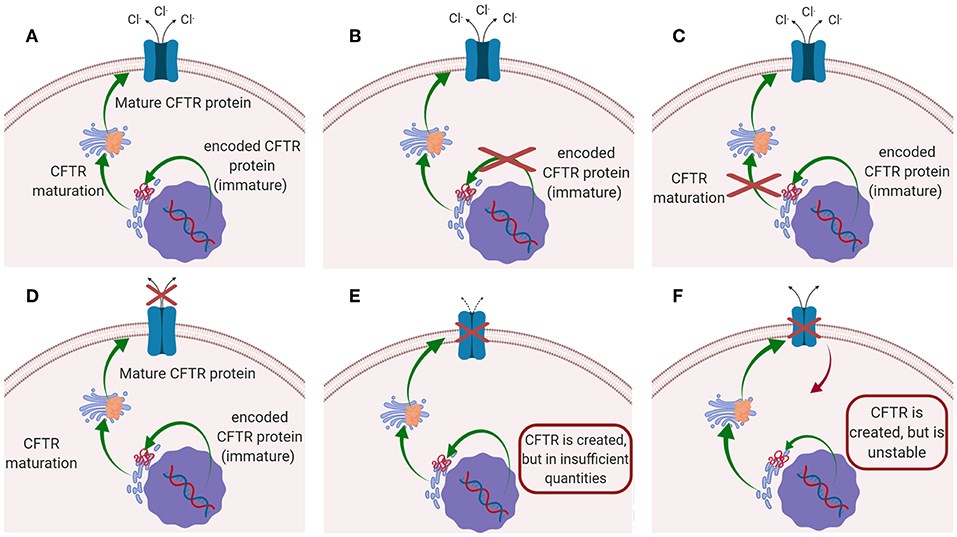 Fig.1 The CFTR mutation type that may lead to CF.1,3
Fig.1 The CFTR mutation type that may lead to CF.1,3
Cystic fibrosis is caused by mutations in the CFTR gene, which result in the production of non-functional CFTR protein or its complete absence. Acting as an ion channel on the cell membrane, this protein regulates the flow of chloride and bicarbonate ions. When it malfunctions, mucus thickens—most notably in the lungs, pancreas, and other organs. However, the pathogenesis is more complex than a simple mutation: as published data shows, the functional effects of CFTR also depend on an extensive network of protein-protein interactions. Disruptions to these interactions can impair the protein's folding, trafficking, and overall stability, offering additional therapeutic targets beyond the gene itself.
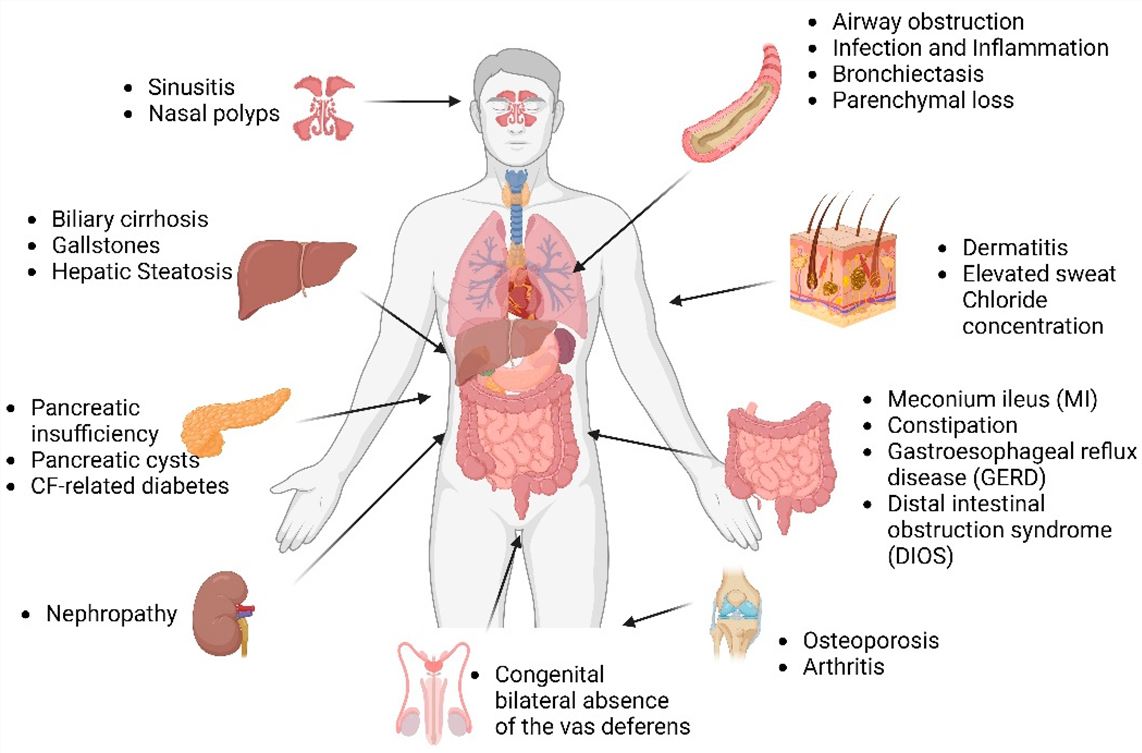 Fig.2 Multi-organ disease manifestations in cystic fibrosis.2,3
Fig.2 Multi-organ disease manifestations in cystic fibrosis.2,3
Therapeutic Targets
For cystic fibrosis (CF), the primary therapeutic goal is to restore functional CFTR protein. One way to achieve this is through gene replacement—delivering a correct copy of the CFTR gene to target cells. Beyond that, advanced strategies also focus on the proteins that interact with CFTR, with the aim of enhancing its function, stability, or proper localization within cells. The lungs remain the key target for treatment, as progressive lung disease is the top cause of death in most CF patients.
Treatment Methods related to AAV
Adeno-associated viruses (AAVs) are an exceptionally promising tool in gene therapy, thanks to their low immunogenicity and ability to deliver genetic material to specific cell types. As a type of nanocarrier, they are well-equipped to overcome the lung's physiological barriers—such as thick mucus and immune system defenses. Recombinant AAV vectors are engineered to carry a functional copy of the CFTR gene, which is then delivered to epithelial cells in the airways. This approach offers a potential long-term solution by providing a stable, functional gene copy, eliminating the need for frequent treatments.
Workflow
-
Required Starting Materials: We need details on your target gene (e.g., CFTR cDNA sequence), desired target tissue/cells (e.g., airway epithelial cells), and any specific promoters/regulatory elements.
- Vector Design and Engineering: Specialists design rAAV vectors per project needs, selecting optimal serotypes and engineering expression cassettes (promoter, transgene, regulatory sequences).
- Plasmid Construction and Quality Control: Synthesize and clone vectors into transfer plasmids; verify integrity/accuracy via restriction enzyme digestion and Sanger sequencing.
- Vector Production and Purification: Produce high-titer rAAV via mammalian cell triple transfection; purify using advanced chromatography to remove impurities/empty capsids.
- Functional Validation and Characterization: Validate transduction efficiency/transgene expression; characterize titer and purity for a verified therapeutic candidate.
- Final Deliverables: Project summary report (raw data, QC/characterization results) and high-purity rAAV sample.
- Estimated Timeframe: 8-12 weeks, variable based on gene complexity and regulatory element needs.
What we can offer
At Creative Biolabs, we empower your research with a comprehensive, end-to-end solution for rAAV-based gene therapy against Cystic Fibrosis. Our offerings are meticulously designed to provide you with the control and quality assurance needed to drive your project forward with confidence.
Customized rAAV Design and Engineering
We provide a one-stop service, tailoring every aspect of your vector from initial design to final production. This includes selecting the optimal serotype, optimizing codon usage of the CFTR gene for maximal expression, and customizing promoters to ensure highly targeted expression in airway epithelial cells.
Efficient Upstream and Downstream Processes
Our well-established workflow covers the entire process, from efficient vector production in our advanced facilities to high-purity downstream purification that significantly reduces empty capsids and minimizes the host immune response.
Comprehensive Quality Control
We apply a robust, Quality-by-Design (QbD) approach and process analytical techniques (PAT) throughout our workflow. We employ high-standard quality control tools to quantify and evaluate the purity, integrity, and stability of our vectors, providing you with verifiable data at every step.
GMP-Compliant Production
Our production processes adhere to the highest standards of Good Manufacturing Practice (GMP) with strict aseptic verification procedures, ensuring the integrity of your therapeutic candidate from the lab bench to clinical trials.
Proven Strain Stability and Yield Optimization
We guarantee the stability of our vectors in cell banks and large-scale productions. Our team optimizes culture conditions to maximize vector yield and functionality, ensuring a consistent and reliable supply for your research.
Experience the Creative Biolabs Advantage - Get a Quote Today
Case Study
| Flow Cytometry |
|---|
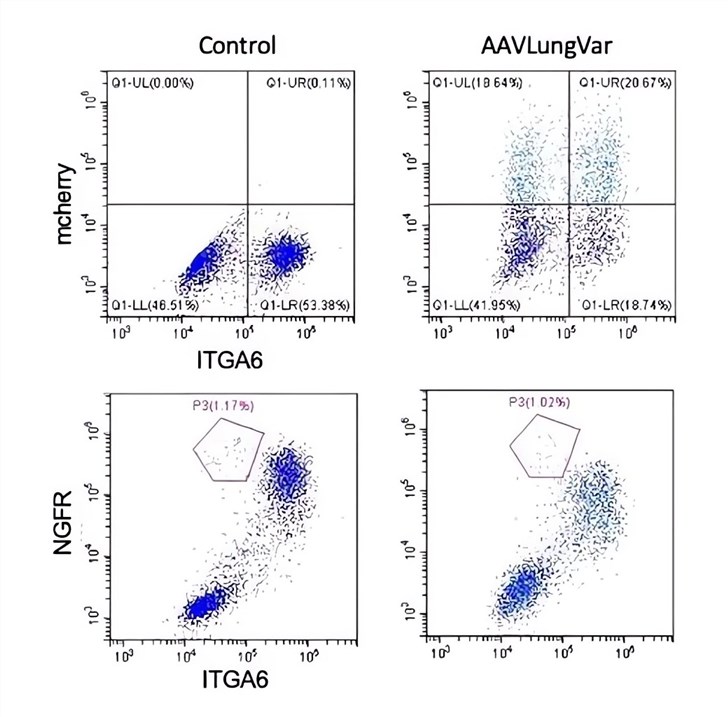
|
| Fig.3 The basal cell markers integrin α-6 (ITGA6) or nerve growth factor receptor (NGFR) were detected by flow cytometry.4 |
| AAV Transduction Efficiency |
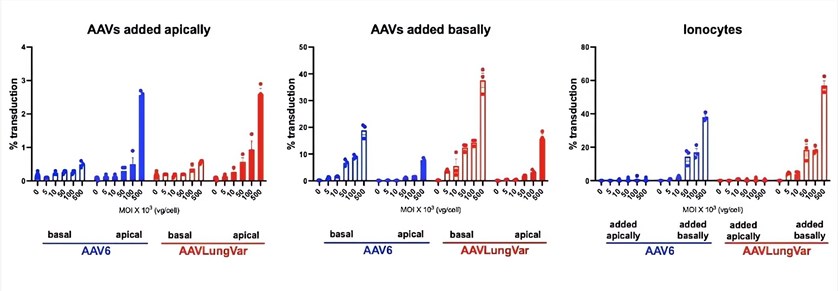
|
| Fig.4 The relationship between AAV concentration and the transduction efficiency of different cells.4 |
| Transduction of AAV In Vivo |
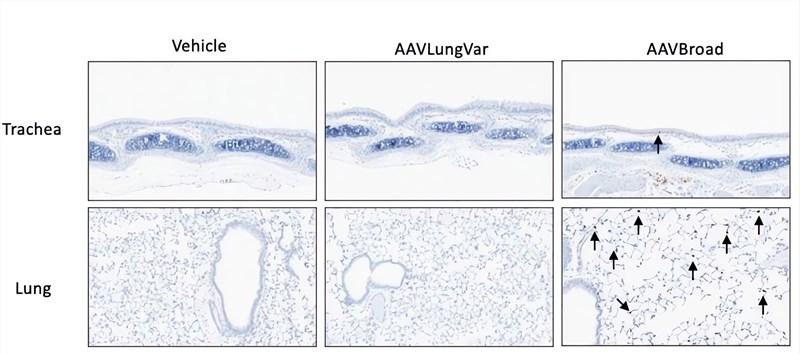
|
| Fig.5 AAV injected into mice through the tail vein has a wide biological distribution.4 |
Customer Reviews

FAQs
What types of genetic material can your rAAV service handle?
Our service is highly flexible and can accommodate a wide range of genetic payloads, including full-length genes like CFTR, as well as smaller genetic elements such as shRNA, miRNAs, or CRISPR components. We work closely with you to ensure your construct is optimized for efficient packaging and expression.
How do you ensure the rAAV vector targets the correct tissue?
We specialize in customizing the AAV capsid to achieve optimal tissue tropism. By selecting from our extensive library of naturally occurring and engineered serotypes, we can design a vector that specifically targets your cells of interest, maximizing therapeutic efficacy while minimizing off-target effects.
What are the quality control measures for your rAAV vectors?
Our rigorous quality control process includes multiple checkpoints to ensure vector purity, titer, and integrity. We perform tests for residual host cell DNA, protein impurities, and empty-to-full capsid ratios. All data is provided in a comprehensive report, so you can have complete confidence in your final product.
How does your rAAV service compare to non-viral delivery methods?
While non-viral methods like liposomes or nanoparticles have their place, rAAV vectors offer distinct advantages, including highly efficient and stable gene expression with minimal risk of insertional mutagenesis. Our expertise in AAV engineering allows us to further enhance these benefits, providing a more robust and reliable solution for long-term gene expression.
Creative Biolabs' custom rAAV design service for cystic fibrosis offers a robust, comprehensive solution for researchers and biopharma companies. Leveraging expertise in vector engineering and deep understanding of disease mechanisms, we create highly specific, pure, and potent therapeutic vectors. Committed to collaborative workflows, we streamline projects and accelerate the journey from concept to clinical reality.
Contact Our Team for More Information and to Discuss Your Project
References
- Velino, Cecilia, et al. "Nanomedicine approaches for the pulmonary treatment of cystic fibrosis." Frontiers in Bioengineering and Biotechnology 7 (2019): 406. https://doi.org/10.3389/fbioe.2019.00406.
- Ramananda, Yashaswini, Anjaparavanda P. Naren, and Kavisha Arora. "Functional consequences of CFTR interactions in cystic fibrosis." International Journal of Molecular Sciences 25.6 (2024): 3384. https://doi.org/10.3390/ijms25063384.
- Distributed under Open Access license CC BY 4.0, without modification.
- Plasschaert, Lindsey W., et al. "A systemically delivered AAV-CFTR gene therapy for cystic fibrosis." bioRxiv (2025): 2025-03. https://doi.org/10.1101/2025.03.20.642115.Distributed under Open Access license CC BY 4.0, figures were cropped.
