AAV Vector Design Services for Other Hereditary Disease
Introduction
For those tackling severe inherited genetic disorders and challenges like low gene expression, vector immunogenicity or inefficient tissue targeting, our custom rAAV design service for other hereditary diseases provides high-quality, application-specific delivery solutions via advanced AAV engineering. This complete, tailored service optimizes vector specificity, boosts transduction efficiency and redirects tropism to enable stable, effective gene expression, delivering validated tools for preclinical and clinical studies.
Discover How We Can Help - Request a Consultation
Hereditary Disease
Hereditary diseases stem from DNA mutations. Though devastating, gene therapy brings new hope—viral vectors like AAVs deliver functional copies of defective/missing genes to address root causes. AAV-mediated gene therapy has great potential, demonstrating its safety and efficacy in preclinical and clinical studies.
Gene therapy represents a promising and transformative approach for a wide range of inherited diseases that lack effective conventional treatments.
- X-linked Duchenne Muscular Dystrophy (DMD): This severe neuromuscular disease is caused by the absence of the dystrophin protein. Gene therapy for DMD involves using AAV vectors to deliver a shortened but functional micro-dystrophin gene. Our services can assist researchers in optimizing vectors to efficiently target muscle cells, including the heart, which is critically affected by cardiomyopathy in DMD patients.
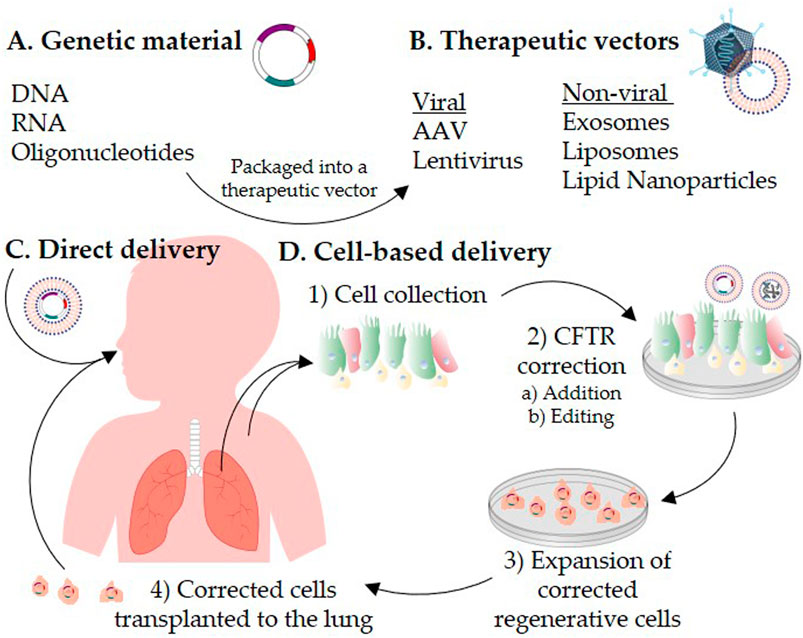 Fig.1 AAV is used in laboratories and clinical Settings for DMD gene therapy, mainly based on the delivery of shortened small or micro dystrophins packaged within adeno-associated viruses.1,3
Fig.1 AAV is used in laboratories and clinical Settings for DMD gene therapy, mainly based on the delivery of shortened small or micro dystrophins packaged within adeno-associated viruses.1,3
- Adenosine Deaminase (ADA) Deficiency: ADA deficiency is a form of severe combined immunodeficiency (SCID) where a missing enzyme leads to a non-functional immune system. Gene therapy for ADA-SCID involves introducing a functional ADA gene into hematopoietic stem cells, allowing the body to produce a healthy immune system.
- Lipoprotein Lipase Deficiency (LPLD): LPLD is a rare metabolic disorder caused by mutations in the LPL gene. AAV-mediated gene therapy has been explored to deliver a functional copy of the LPL gene, with the goal of restoring lipoprotein lipase activity and normalizing triglyceride levels to prevent acute pancreatitis.
- Development of AAV Vector for Heart Failure Research: While often a complication of other diseases like DMD, primary heart failure is also a target for gene therapy. AAV vectors are being investigated to deliver genes that can restore normal cardiac function, address calcium handling issues, and protect against adverse remodeling of the heart.
- Development of AAV Vector for Rheumatoid Arthritis Research: AAV vectors are a promising tool for treating localized inflammatory conditions. For rheumatoid arthritis, research focuses on using AAVs to deliver anti-inflammatory genes directly to affected joints, offering a single, long-lasting treatment to reduce inflammation and prevent joint damage.
- Cystic Fibrosis (CF): This life-threatening disorder is caused by mutations in the CFTR gene. Researchers are using AAV vectors to deliver a functional CFTR gene to the airways, with the goal of restoring fluid balance and clearing the thick, sticky mucus that characterizes CF lung disease.
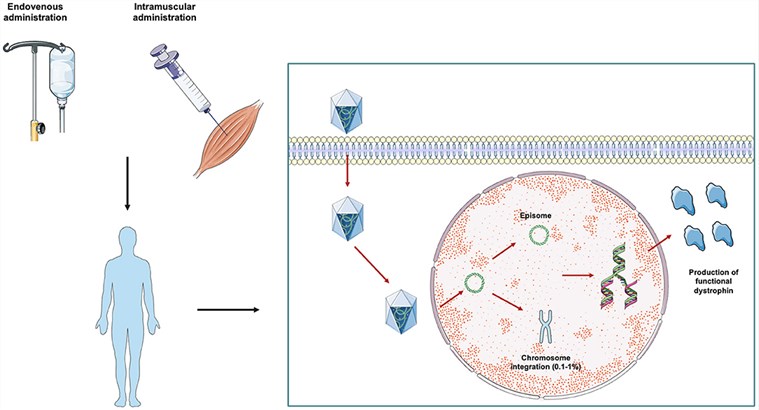 Fig.2 AAV can be used for the treatment of cystic fibrosis.2,3
Fig.2 AAV can be used for the treatment of cystic fibrosis.2,3
Workflow
- Required Starting Materials: To initiate a project, clients typically provide a few key materials, including the sequence of the therapeutic gene, details of the target cell or tissue type, and the desired expression level or promoter. This information allows us to precisely engineer a vector for your unique application.
- Vector Design and Optimization: Experts design rAAV vectors per your materials, selecting optimal serotype, promoter, and regulatory elements to boost expression and reduce off-target effects.
- Gene Synthesis and Vector Construction: Synthesize therapeutic genes and clone into optimized AAV backbones, ensuring correct orientation and functionality.
- AAV Production and Purification: Manufacture and purify via high-titer platforms, yielding research-grade vectors for in vitro/in vivo studies.
- Quality Control and Validation: Rigorous batch testing (qPCR, silver staining) for titer, purity, and integrity to meet high standards.
- Functional Analysis (Optional): Custom functional assays available to verify gene expression and transduction efficiency in specified cell lines.
- Final Deliverables: Purified AAV vectors, detailed QC report (full characterization), and project summary (design/production process).
- Estimated Timeframe: 8–16 weeks, dependent on vector design complexity and custom validation needs.
What We Can Offer
At Creative Biolabs, we provide a robust, end-to-end gene therapy service designed to meet the unique needs of your research and development projects. Our expertise in vector engineering, combined with our state-of-the-art facilities, allows us to deliver high-quality, customized solutions for treating a variety of hereditary diseases.
Customized Vector Design and Optimization
No one-size-fits-all—we collaborate to tailor rAAV vectors (serotype selection, promoter matching for target cells) for maximum transduction efficiency and stable gene expression.
Scalable, High-Titer AAV Production
Advanced platforms scale from research to preclinical/clinical volumes. Industrial fermenters and robust quality systems ensure consistent, high-yield supply.
Rigorous Quality Control and Validation
Strict QbD approach with PAT; every batch undergoes rigorous testing (purity, titer, integrity) to guarantee reliability.
Expert Consultation and Support
Seasoned biologists offer end-to-end support—navigating challenges, optimizing protocols, and keeping projects on track.
GMP-Compliant Processes
Adhere to GMP principles, meeting top industry standards for quality and safety, ideal for clinical-oriented projects.
Customer Reviews

FAQs
How do you ensure the AAV vectors are safe and not immunogenic?
We use non-pathogenic, replication-incompetent AAV vectors. Our advanced engineering techniques focus on selecting the most suitable serotypes and optimizing the vector design to minimize the risk of an immune response, maximizing the safety and efficacy of your study.
Can you customize the AAV vector for my specific gene and cell type?
Absolutely. Our service is built around custom design. We work closely with you to select the ideal AAV serotype, promoter, and other elements to ensure the vector is optimized for your target gene and cell type, maximizing transduction efficiency and expression.
How does your service compare to off-the-shelf AAV products?
While off-the-shelf products can be a starting point, they are not optimized for specific applications. Our custom service provides a purpose-built solution that addresses your unique challenges, leading to higher success rates, more reliable data, and ultimately, faster project completion.
How long does the AAV vector's effect last?
The duration of expression is a critical consideration. While it can vary depending on the specific application and target tissue, AAV vectors are known for their ability to provide long-term gene expression in many cell types, which is one of their key advantages for gene therapy.
Creative Biolabs' custom rAAV design service for Other Hereditary Disease provides a powerful and precise tool for advancing your research. Our expertise in vector engineering and our commitment to quality ensure that your projects are built for success. We invite you to leverage our capabilities to overcome your most complex scientific challenges.
Contact our team for a detailed discussion about your project
References
- Manini, Arianna, et al. "Adeno-associated virus (AAV)-mediated gene therapy for Duchenne muscular dystrophy: the issue of transgene persistence." Frontiers in neurology 12 (2022): 814174. https://doi.org/10.3389/fneur.2021.814174.
- Allan, Katelin M., et al. "Treatment of cystic fibrosis: from gene-to cell-based therapies." Frontiers in pharmacology 12 (2021): 639475. https://doi.org/10.3389/fphar.2021.639475.
- Distributed under Open Access license CC BY 4.0, without modification.
