AAV Vector Design Service for Age related Macular Degeneration
Introduction
Age-related macular degeneration (AMD) is a progressive retinal disease and top cause of irreversible blindness. At Creative Biolabs, we aim to replace burdensome frequent injections with our custom rAAV vector service for AMD. Leveraging advanced recombinant DNA and protein engineering, we provide single-administration vectors enabling sustained protein expression, delivering durable efficacy to transform wet and dry AMD treatment.
Age Related Macular Degeneration (AMD)
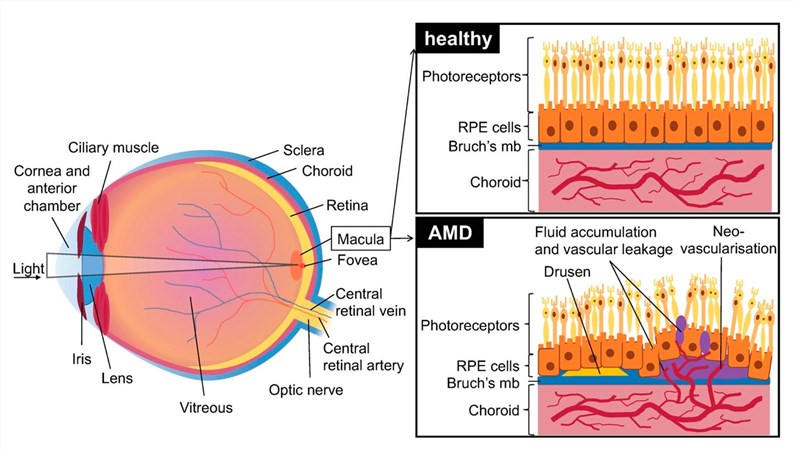 Fig.1 Schematic diagram of healthy eyes with retinal, choroidal and retinal pigment epithelial (RPE) cells, as well as the impact of AMD.1
Fig.1 Schematic diagram of healthy eyes with retinal, choroidal and retinal pigment epithelial (RPE) cells, as well as the impact of AMD.1
Pathogenesis
Dry AMD (Atrophic AMD)
As the predominant form of AMD (accounting for 85%-90% of cases), its core pathological mechanism is closely linked to chronic oxidative stress and inflammatory responses. Metabolic waste products (known as drusen) gradually accumulate beneath the retina, triggering the progressive atrophy and functional degeneration of retinal pigment epithelial (RPE) cells and photoreceptors (key cells responsible for light sensing). This ultimately leads to the gradual decline of central vision.
Wet AMD (Neovascular AMD)
Although it accounts for only 10%-15% of AMD cases, it poses a much higher risk of blindness. Its pathological hallmark is choroidal neovascularization: after oxidative stress and inflammation disrupt the blood-retinal barrier, abnormal new blood vessels grow beneath the retina. These fragile vessels are prone to leaking fluid or blood, causing macular edema and structural damage, which results in rapid and severe vision loss.
Ttherapeutic Approaches
Treatments for Dry AMD
- Basic interventions: Antioxidant supplementation (e.g., vitamin C/E, lutein, zeaxanthin) plus lifestyle adjustments (blood pressure/glucose control, avoiding strong light, quitting smoking).
- Emerging directions: AAV gene therapy (delivers complement system-regulating genes or uses gene-editing for neuroprotection) and retinal cell transplantation (under clinical exploration).
Treatments for Wet AMD
- Core goal: Inhibit neovascularization and reduce leakage.
Traditional therapy: Regular intravitreal anti-VEGF injections (e.g., ranibizumab, aflibercept; monthly/bi-monthly) with/without photodynamic therapy. Frequent injections may lower compliance.
Breakthrough therapy: AAV gene therapy (single administration) delivers anti-VEGF-encoding genes to enable continuous intraocular protein expression. Leverages the eye's immune privilege and AAV's low immunogenicity/long-term expression.
Workflow
Required Starting Materials: To initiate a project, clients typically provide a target gene sequence (e.g., an anti-VEGF construct, a neuroprotective factor, or a complement inhibitor), the desired expression profile (e.g., constitutive or inducible expression), and information on the target cell type (e.g., retinal pigment epithelial cells or photoreceptors).
Key Steps Involved:
- Initial Consultation & Design: Discuss therapeutic goals, then develop customized vector strategies—including serotype selection and promoter optimization for precise ocular targeting and appropriate expression.
- Vector Construction & Engineering: Synthesize therapeutic genes and expression cassettes; use capsid engineering/rational design to boost tropism and reduce immune responses (backed by research).
- Vector Production & Purification: Produce high-titer, high-purity rAAV with low empty capsids via advanced, scalable manufacturing (consistent for preclinical/clinical use).
- Characterization & QC: Conduct rigorous testing (titer, sterility, identity) plus in vitro/in vivo studies to confirm transduction efficiency and sustained transgene expression.
- Final Deliverables: Provide fully characterized, lead-ready rAAV, comprehensive process/QC reports, and stability analysis for downstream research.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 16 weeks, depending on the complexity of the vector design, the scope of optimization, and the specific QC requirements.
What We Can Offer
Customized rAAV Vector Design
Creative Biolabs offers a bespoke service for Age-Related Macular Degeneration (AMD) gene therapy, designing vectors specifically for long-term, stable transgene expression in targeted retinal cells. This includes serotype selection, promoter optimization, and immunogenicity mitigation tailored to your unique therapeutic goals.
Full-Spectrum Service
We provide a one-stop service that takes your project from initial design to a lead-ready therapeutic vector, offering a comprehensive workflow from concept to a lead candidate.
GMP-Grade Production
Our scalable, state-of-the-art manufacturing platform ensures high-titer, high-purity rAAV vectors with low empty capsid ratios, guaranteeing consistency from early-stage research to clinical trials.
Rigorous Quality Control
Every vector undergoes comprehensive characterization and stringent quality control (QC) testing, including titer, purity, sterility, and in vitro functional assays, ensuring your product is compliant with regulatory standards.
Innovation and Expertise
Our approach is built on advanced recombinant DNA technology and innovative protein engineering, backed by a foundation of published data and deep scientific expertise in mitigating pre-existing immunity and optimizing AAV capsids for ocular delivery.
Discover How We Can Help - Request a Consultation
Customer Reviews

Experience the Creative Biolabs Advantage - Get a Quote Today
FAQs
How does gene therapy for AMD compare to traditional anti-VEGF injections?
Gene therapy provides a single, long-lasting treatment that allows the eye to produce its own therapeutic protein, such as an anti-VEGF agent. This can potentially eliminate the need for frequent injections, which is a significant burden for patients and can lead to inconsistent therapeutic levels. Our approach is designed to provide sustained, stable protein expression for prolonged therapeutic effect.
Is it possible to use your service to target a specific cell type in the retina?
Absolutely. Our vector engineering strategies are highly customizable. We utilize a variety of AAV serotypes and promoters to achieve precise and efficient transduction of specific ocular cells, such as retinal pigment epithelial cells, photoreceptors, or Müller glial cells, ensuring your therapeutic payload is delivered exactly where it's needed.
What measures do you take to ensure the safety and quality of the gene therapy vector?
We adhere to the highest quality standards. Our vectors are produced under stringent quality control measures, including comprehensive purity and potency assays. We also engineer our vectors to be as safe as possible, addressing potential issues like off-target effects and immunogenicity through rational design and rigorous testing.
At Creative Biolabs, we pioneer Age-Related Macular Degeneration gene therapy. Leveraging AAV vector engineering expertise and a client-centric approach, we offer end-to-end services from custom vector design to production and characterization, supporting the development of durable, single-administration therapies that preserve vision.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Rastoin, Olivia, Gilles Pagès, and Maeva Dufies. "Experimental models in neovascular age related macular degeneration." International journal of molecular sciences 21.13 (2020): 4627. https://doi.org/10.3390/ijms21134627. Distributed under Open Access license CC BY 4.0, without modification.
