AAV Vector Design Services for Ocular Disease
Introduction
Facing challenges in developing effective ocular gene therapies, such as vector design issues or optimizing delivery to specific retinal cells, our custom rAAV service accelerates discovery and development via advanced recombinant AAV technology and tailored engineering. At Creative Biolabs, we provide bespoke rAAV solutions for ocular therapy, addressing eye-specific delivery hurdles to enable efficient, targeted transduction for retinal disorders, plus optimized vectors, preclinical support and streamlined development.
Discover How We Can Help - Request a Consultation
Ocular Disease
Ocular gene therapy is a fast-growing field for treating vision loss, with the eye's immune-privileged, accessible anatomy ideal for local delivery. AAV vectors lead the field for their safety, broad transduction ability, and long-term expression. Our services use this technology to deliver tailored solutions for your research.
Creative Biolabs expertise in rAAV design extends to a wide range of inherited and acquired ocular diseases. Our customized vectors can be engineered to target the specific cell types affected by conditions such as:
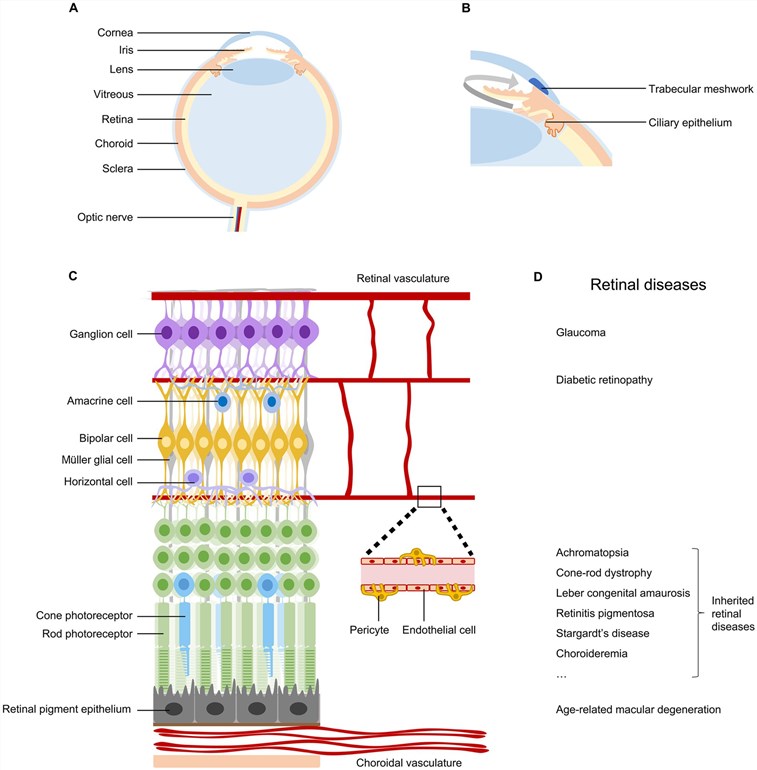 Fig.1 The anatomical structure of the retina and common eye diseases.1
Fig.1 The anatomical structure of the retina and common eye diseases.1
Age-Related Macular Degeneration (AMD)
AMD is a progressive condition affecting the macula, the central part of the retina responsible for sharp, central vision. It's the leading cause of irreversible vision loss in people over 60. There are two main forms: dry AMD, characterized by the breakdown of light-sensitive cells, and wet AMD, involving abnormal blood vessel growth under the retina. Gene therapy for wet AMD can deliver genes that produce anti-angiogenic factors, effectively providing a long-term, single-dose treatment to inhibit this neovascularization.
Achromatopsia
Achromatopsia is a group of rare, inherited retinal disorders characterized by the absence of functional cone photoreceptor cells. This results in severe vision impairment from birth, including poor visual acuity, extreme light sensitivity (photophobia), and a complete lack of color vision. The condition is often caused by mutations in genes such as CNGA3 and CNGB3. Gene therapy aims to deliver a healthy copy of the mutated gene to the remaining, non-functional cone cells to restore their function.
Choroideremia
Choroideremia is a rare, X-linked genetic disorder that causes progressive vision loss in males. It is caused by a mutation in the CHM gene, which leads to the degeneration of the choroid, retinal pigment epithelium (RPE), and photoreceptor cells. This degeneration starts in the periphery of the retina and slowly moves inward, leading to tunnel vision and eventually complete blindness. Gene therapy for choroideremia involves delivering a healthy CHM gene to the retinal cells to halt the degeneration.
Leber Congenital Amaurosis (LCA)
LCA is a group of inherited retinal diseases that cause severe vision loss from infancy. It is one of the most common genetic causes of childhood blindness. LCA is linked to mutations in over 25 different genes, including RPE65, which was the target of the first FDA-approved gene therapy for an inherited disease. Gene therapy for LCA seeks to provide a working copy of the mutated gene to prevent or slow the degeneration of the photoreceptor cells.
Retinitis Pigmentosa (RP)
RP is a group of inherited eye diseases that cause slow, progressive vision loss. It is characterized by the breakdown of cells in the retina (photoreceptors) over time. Symptoms typically begin with difficulty seeing at night and a gradual loss of peripheral (side) vision. Over 100 different genes can be mutated to cause RP, making it a highly complex target for gene therapy. The goal of gene therapy for RP is to introduce a functional gene to a subset of retinal cells to either slow the progression of the disease or restore some function to the affected photoreceptors.
Workflow
-
Required Starting Materials:
- Target Gene Information: Full-length cDNA sequence, desired promoter elements, and any specific regulatory sequences.
- Disease Model Details: Information on the specific ocular disease, target cell types (e.g., photoreceptors, RPE, ganglion cells), and relevant animal models if applicable.
- Project Goals: Desired therapeutic outcome, expression duration, and any specific safety or immunogenicity considerations.
-
Key Steps Involved:
-
Project Consultation & Design Strategy:
Our expert team collaborates with you to define project scope, discuss target biology, and identify the most suitable AAV serotypes and vector configurations for your specific ocular disease. This involves reviewing existing literature and client-provided data. -
rAAV Vector Construction & Optimization:
Based on the design strategy, we synthesize and clone the therapeutic gene into the optimized rAAV backbone. This step includes codon optimization, incorporation of tissue-specific promoters, and addition of enhancer elements for enhanced expression in target ocular cells. -
Large-Scale rAAV Production & Purification:
We employ state-of-the-art HEK293-based triple transfection systems for high-titer rAAV production. This is followed by robust purification methods (e.g., chromatography-based) to ensure high purity and removal of empty capsids. -
In Vitro & In Vivo Characterization:
For in vitro studies, we assess vector transduction efficiency and gene expression in relevant ocular cell lines. For in vivo studies, we can perform initial assessments in animal models (e.g., rodents) to evaluate biodistribution, expression, and preliminary efficacy. -
Quality Control & Documentation:
Rigorous QC checks are performed at each stage, including titering, purity analysis (SDS-PAGE, DLS), aggregation assessment, and endotoxin testing. Comprehensive documentation is provided.
-
Project Consultation & Design Strategy:
-
Final Deliverables:
- High-titer, ultra-pure rAAV viral vectors ready for research or preclinical studies.
- Detailed vector characterization reports including titer, purity, and integrity data.
- Comprehensive project summary and experimental protocols used during the design and production phases.
-
Estimated Timeframe:
The typical timeframe for this service ranges from 8 to 16 weeks, depending on the complexity of the vector design, the specific serotype chosen, and the scope of characterization required.
What We Can Offer
At Creative Biolabs, our commitment to your success in ocular gene therapy is demonstrated through our comprehensive and flexible service offerings.
Customized Vector Design
We offer a one-stop solution from initial consultation to final delivery, with every aspect of the rAAV vector—including serotype selection, promoter optimization, and gene cassette design—customized to your specific research goals and target cell types.
Scalable Production
Our advanced production platform enables us to scale your project from laboratory-scale proof-of-concept studies to large-scale, high-titer production batches for preclinical in vivo studies, ensuring a seamless transition as your research progresses.
Efficient Process Development
We employ established process development techniques to optimize both upstream (vector production) and downstream (purification) processes, guaranteeing maximum yield, purity, and consistency for your final product.
Robust Quality Control
We have implemented a well-established quality system and employ advanced process analytical techniques (PAT) to ensure the stability and integrity of the rAAV vectors. Our strict aseptic verification procedures throughout the production process ensure a high-quality product.
Expert Consultation
Our team of experienced scientists provides expert guidance on all aspects of your project, from optimizing codon usage for enhanced expression in specific cell types to selecting the most effective route of administration.
Comprehensive Documentation
We provide high-standard quality control reports and a complete history of the production process, essential for supporting research publications and future regulatory filings.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How do you ensure the tropism of your AAV vectors for specific ocular cell types?
We utilize a variety of naturally occurring and engineered AAV serotypes known for their specific tropism to different retinal cells (e.g., AAV2 for ganglion cells, AAV8 and AAV5 for photoreceptors). Additionally, we can incorporate specific promoters to ensure gene expression is limited to the intended cell type, maximizing therapeutic efficacy while minimizing off-target effects. For more details on serotype selection, please contact our specialists.
What quality control measures are in place for your rAAV products?
Our rAAV production follows a rigorous quality control process that includes titering by qPCR, purity analysis via SDS-PAGE and silver staining, aggregation measurement with Dynamic Light Scattering (DLS), and sterility and endotoxin testing. This ensures our vectors are of the highest quality for your preclinical and research applications. A full Certificate of Analysis is provided with every order.
Is your service suitable for both basic research and preclinical studies?
Absolutely. Our services are designed to be highly flexible. We can produce small-scale, research-grade batches for initial proof-of-concept studies and also offer large-scale, high-purity production suitable for preclinical in vivo studies. We can also help with documentation to support your regulatory filings.
Can you help with the choice of gene and promoter?
Yes, our scientific team has extensive experience in ocular biology and gene therapy. We can provide consultation on the most effective promoters and assist in optimizing your gene of interest for enhanced expression and stability. The final decision is always yours, but our guidance can help you avoid common pitfalls.
How does your service compare to a DIY approach for AAV production?
While in-house production is an option, it often requires significant time, specialized equipment, and can yield variable quality. Our service provides a streamlined, validated workflow with guaranteed high-titer and high-purity vectors, saving you valuable time and resources. We handle the technical complexities so you can focus on your research.
Creative Biolabs is your dedicated partner in advancing ocular gene therapy. Our comprehensive service, from custom vector design to high-quality production, is built on a foundation of scientific excellence and a deep understanding of retinal biology. We are committed to helping you accelerate your research and translate your discoveries into impactful therapies for vision-saving treatments.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Xia, Xue, and Xinzheng Guo. "Adeno-associated virus vectors for retinal gene therapy in basic research and clinical studies." Frontiers in Medicine 10 (2023): 1310050. https://doi.org/10.3389/fmed.2023.1310050. Distributed under Open Access license CC BY 4.0, without modification.
