Rational Design based AAV Capsid Amino Acid Mutation Service
Introduction
For projects troubled by long development cycles and suboptimal gene delivery efficiency, our Rational Design-Based Capsid Amino-acid Mutation service addresses these pain points. Via advanced capsid engineering, it overcomes biological barriers and improves tissue tropism to accelerate research and deliver targeted viral vectors. Creative Biolabs provides clear, step-by-step solutions with highly customized AAVs, ensuring higher transduction efficiency, superior tissue specificity, and reduced immunogenicity risk. We deliver data-driven results essential for preclinical and clinical success.
Discover How We Can Help - Request a Consultation
Rational Design-Based Capsid Amino-acid Mutation
Rational design-based AAV capsid amino acid mutation is a precise, goal-oriented genetic engineering strategy. It relies on existing knowledge of AAV capsid structure, function, and host interaction mechanisms to specifically alter targeted amino acid residues, optimizing AAV vector performance for applications like gene therapy. Unlike directed evolution (dependent on random mutation and screening), it follows "understanding before modifying" to design predictable mutations based on biological and structural data.
Core Design Bases and Objectives
- Tropism Modification
Tropism Modification is based on the fact that specific amino acid residues on the AAV capsid surface directly determine its binding ability to host cell surface receptors (the core of tissue tropism), to mutate these key residues to weaken or eliminate natural receptor binding (and potentially introduce new binding sites), thereby enabling precise targeting of specific tissues or cells and reducing off-target effects.
- Immunogenicity Optimization
Immunogenicity Optimization is grounded in pre-existing human immunity to AAV targeting capsid immunodominant epitopes (which neutralize vectors and reduce efficacy), aiming to identify and mutate amino acid residues recognized by neutralizing antibodies to alter immunogenic epitope structure, thus evading or reducing host immune recognition and attack.
- Transduction Efficiency Enhancement
Transduction Efficiency Enhancement stems from AAV transduction efficiency—related to capsid-receptor binding and viral nuclear transport post-entry—being tied to specific amino acid sequences, with the goal of mutating to optimize capsid spatial conformation, enhancing receptor binding affinity or intracellular transport efficiency to boost target gene expression.
Key Technical Steps
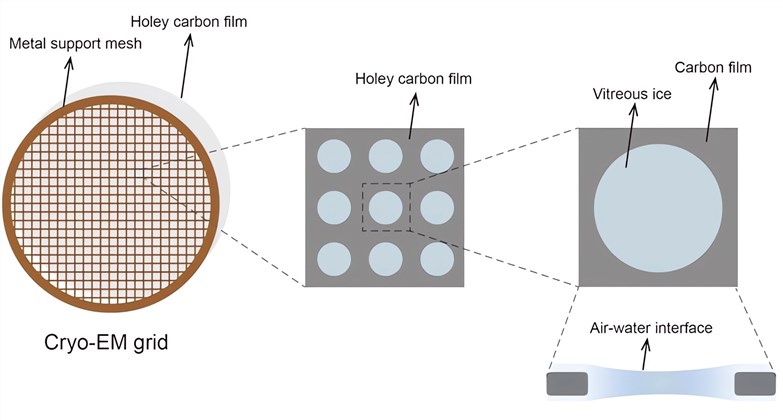
- Structure and Function Analysis: High-resolution 3D structures of AAV capsids are resolved using techniques such as X-ray crystallography and cryo-electron microscopy (Cryo-EM). Combined with bioinformatic analysis, key amino acid sites related to receptor binding, immune recognition, and nuclear localization are identified.
| X-ray crystallography |
|---|
|
|
| Cryo-Electron Microscopy (Cryo-EM) |
|
|
- Mutant Design: Based on the above analysis, specific mutation schemes (e.g., point mutations to replace single amino acids, insertion mutations to introduce short peptide sequences, or deletion mutations to remove specific fragments) are designed using molecular biology principles.
- Vector Construction and Production: The designed mutations are introduced into the coding gene of AAV capsid proteins via gene cloning to construct recombinant AAV vectors, which are then produced and purified in suitable cell systems.
- Functional Validation and Optimization: The mutated AAV vectors are comprehensively characterized, including testing of targeting specificity, transduction efficiency, immunogenicity, and vector stability. Based on validation results, mutation sites and methods can be iteratively optimized.
Advantages
The key advantages of our rational design service are precision, control, and efficiency. By directly modifying the capsid, we can create vectors with enhanced properties tailored to your exact needs, bypassing the time-consuming and often unpredictable nature of random screening.
Workflow
- Required Starting Materials:
- Target Gene Information: Your gene of interest, including the sequence and any specific expression requirements.
- Target Tissue or Cell Line: Clear identification of the specific cells, tissues, or organs you need to target.
- Project Objectives: A detailed description of your research goals and desired outcomes.
- Project Assessment & Design: We comprehensively analyze your project goals and materials, leveraging AAV biology expertise to design a rational capsid mutation strategy and identify target residues for modification.
- Capsid Variant Generation: Advanced molecular biology techniques are used to create an AAV capsid variant library, with precise amino acid substitutions for desired traits like improved tropism or immune evasion.
- In Vitro & In Vivo Screening: Variants are rigorously screened in vitro and in vivo to evaluate performance, identifying candidates with optimal transduction efficiency and target tissue specificity.
- Characterization & Optimization: Top candidates undergo detailed characterization (immunogenicity, stability, off-target effects), with data used to refine and optimize the final vector.
- Final Vector Production & Quality Control: We scale up production of the lead candidate for clinical-grade vectors, which undergo rigorous QC checks for purity, titer, and potency.
-
Final Deliverables:
- A fully characterized, high-titer AAV vector optimized for your specific application.
- A detailed technical report outlining the design, screening results, and quality control data.
- The final vector sequence includes the specific amino acid mutations.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 12 weeks, depending on the complexity of the project and the specificity of the required vector.
What We Can Offer
Customized Service
We focus on custom Rational Design-Based Capsid Amino-acid Mutation, with no pre-made products. Each project is uniquely designed for your research needs and target tissue gene delivery optimization.
Rational Design-Based Services
We offer comprehensive services via our rational design platform—AAV Serotype Switching, Targeting Ligand Insertion, Immune Evasion Engineering—each tailored to solve specific vector performance challenges.
Precision and Efficiency
AAV capsid biology expertise plus cutting-edge bioinformatics enable precise mutations, slashing time and resources for vector optimization vs. traditional random mutagenesis.
Seamless Integration
Holistic service covers project assessment, capsid design, vector production to QC, ensuring consistent, reliable workflow at every stage.
Expert Support
Our AAV specialists collaborate closely to understand your goals, guiding you throughout for a successful outcome.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How is rational design different from directed evolution?
Rational design is a targeted approach where we make specific changes based on our knowledge of AAV biology. Directed evolution is a more unbiased approach that creates large libraries of variants and selects for the best performers. We combine both methods to provide the most comprehensive solution.
What types of projects is this service best for?
Our service is ideal for projects requiring highly specific gene delivery to difficult-to-transduce tissues, or for therapies where pre-existing immunity is a major concern.
How do you ensure the quality of the final vector?
Every vector undergoes a strict quality control process, including analyses for purity, titer, and potency, to ensure it meets our high standards and is ready for your research.
Can your service help if I already have a lead candidate?
Absolutely. We can take your lead candidate and use our rational design platform to further optimize its performance, for example, by improving its stability or reducing its immunogenicity.
The future of gene therapy is in precise, targeted delivery. Our integrated capsid engineering services offer the solution you need to accelerate your research and move your therapeutic candidates toward the clinic. Contact our team to discover how our unique approach can benefit your project.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Xu, Yixin, and Shangyu Dang. "Recent technical advances in sample preparation for single-particle cryo-EM." Frontiers in molecular biosciences 9 (2022): 892459. https://doi.org/10.3389/fmolb.2022.892459. Distributed under Open Access license CC BY 4.0, this figure was cropped.