Peptide Insertion Service for Advanced AAV Vector Cell Surface Targeting
Introduction
Peptide Insertion for Cell Surface Targeting of Advanced AAVs Vector is a core capsid engineering technology. It inserts cell-specific ligand peptides into exposed variable regions of AAV capsids, enabling "ligand-receptor" specific binding to redirect vectors to target cells/tissues, boosting transduction efficiency while reducing off-target effects.
Creative Biolabs' service excels in this field: it offers tailored peptide selection (from phage libraries or custom design) and optimized insertion sites to fit diverse needs. Rigorous validation ensures capsid stability and targeting precision, delivering high-yield, functional vectors that streamline research and accelerate pre-clinical progress.
Discover How We Can Help - Request a Consultation
Peptide Insertion for Cell Surface Targeting of Advanced AAVs Vector
Core Principle
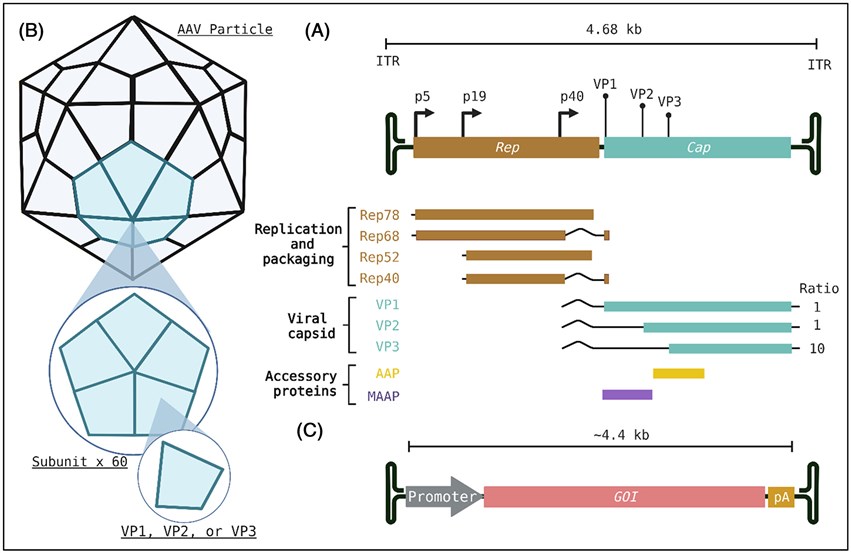 Fig.1 The basic structure and genome of AAV, an icosahedral AAV capsid composed of 60 subunits, are made up of viral proteins VP1, VP2, and VP3.1
Fig.1 The basic structure and genome of AAV, an icosahedral AAV capsid composed of 60 subunits, are made up of viral proteins VP1, VP2, and VP3.1
- Modifiability Basis of AAV Capsid: The AAV capsid (assembled from VP1/VP2/VP3) has surface Variable Regions (VRs). These regions have high amino acid plasticity without damaging capsid stability, serving as ideal sites for exogenous peptide insertion.
- Core Logic of Targeting: Receptor-specific ligand peptides (e.g., tumor-targeting RGD, nerve-targeting RVG) are genetically inserted into capsid VRs. The modified capsid binds target cell receptors via "ligand-receptor" interactions, mediating viral entry for targeted delivery.
Key Design and Implementation Strategies
Targeting peptides are derived from natural ligands like cytokine fragments, phage display peptide library screening, and computer-aided design. They need high specificity—binding only to target cell receptors, high affinity with strong binding capacity, and short sequences to avoid disrupting capsid assembly. Examples include RGD peptide targeting integrin αvβ3 on tumor cells, TAT peptide crossing the blood-brain barrier to target nerve cells, and transferrin receptor-binding peptide targeting hepatocytes or nerve cells.
Core Advantages
- Precisely Enhanced Tissue/Cell Targeting: Breaks natural AAV tropism limits (e.g., AAV9 liver accumulation), enabling muscle/CNS targeting and reducing off-target risks.
- Balancing Safety and Activity: Only modifies capsid (no genome changes), retains low immunogenicity and long-term expression; most peptides have no toxic side effects.
- High Customizability: Swappable peptides for needs like tumors or neurodegenerative diseases, enabling flexible "one backbone, multiple targets" design.
Workflow
-
Required Starting Materials: To initiate a project, clients typically provide detailed information on the following:
- The specific cell or tissue type to be targeted.
- The target cell-specific receptors or markers.
- The desired AAV serotype to be used as a base.
- Peptide Design and Selection: Our team designs high-affinity target receptor peptides, leveraging bioinformatic data and rational design for optimal binding and stability.
- AAV Capsid Engineering: Precisely insert selected peptides into strategic AAV capsid variable regions (VRs) to alter tropism while preserving vector integrity.
- Vector Production and Purification: Produce engineered vectors in controlled environments via advanced platforms, ensuring high titer, purity, and batch consistency.
- Validation and Optimization: Rigorously test targeting specificity, transduction efficiency, and performance, with optional in vitro/in vivo validation for application efficacy.
- Final Quality Control: Conduct final batch checks to meet stringent standards for purity, integrity, and functionality prior to delivery.
-
Final Deliverables: Upon project completion, you will receive:
- A validated, ready-to-use AAV vector with enhanced targeting capabilities.
- A comprehensive technical report detailing the design, production, and validation data.
- Supporting documentation for the modified vector, including sequence information.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 14 weeks, depending on the complexity of the peptide design, the serotype used, and the scope of the validation studies requested.
What we can offer
Service Scope
One-stop service from custom peptide design to validated, ready-to-use AAV vectors.
Peptide Selection Platform
Advanced rational design and high-throughput screening platforms for optimal peptide selection.
Targeting Precision
Tailored capsid engineering to achieve precise tropism for your specific cell or tissue targets.
Delivery Efficacy
Guaranteed high transduction efficiency and enhanced gene expression, reducing required vector dosage.
Quality Assurance
Strict quality control and comprehensive analytical testing at every stage to ensure vector integrity and safety.
Technical Expertise
Unmatched expertise in AAV biology and protein engineering, supported by extensive published data.
Serotype Flexibility
Full flexibility to work with a wide range of AAV serotypes to meet your project's needs.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How does peptide insertion improve upon wild-type AAVs for gene delivery?
Wild-type AAVs can have broad tropism, meaning they may transduce cells you aren't trying to target, leading to off-target effects and a less efficient therapeutic outcome. Peptide insertion allows us to engineer the vector to specifically recognize and bind to receptors on your desired cells, dramatically increasing targeting accuracy and transduction efficiency.
Can you help me select the best peptide for my specific cell type?
Absolutely. Our service begins with a thorough consultation where we analyze your target cell and desired application. Our team of experts will then apply bioinformatics and rational design principles to identify and select the optimal peptide sequence to achieve highly specific and efficient targeting for your project.
What are the advantages of peptide insertion compared to other targeting methods, such as using bispecific antibodies?
While bispecific antibodies offer a flexible, modular approach to targeting, peptide insertion provides a permanent, single-component solution. This can simplify the delivery system and may reduce potential immunogenicity concerns associated with a separate antibody component. We can discuss the pros and cons of each approach to help you choose the best strategy for your needs.
How do you ensure the modified AAV vector remains stable and safe for my research?
We use a multi-step quality control process to ensure the integrity of our engineered vectors. Our process includes rigorous testing for vector stability, purity, and functionality. We ensure that the peptide insertion does not compromise the viral capsid's structural integrity or packaging efficiency.
For detailed information and to discuss how our Peptide Insertion for Cell Surface Targeting of Advanced AAVs Vector Service can benefit your specific project, please reach out to our team of experts.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Chuecos, Marcel A., and William R. Lagor. "Liver directed adeno‐associated viral vectors to treat metabolic disease." Journal of inherited metabolic disease 47.1 (2024): 22-40. https://doi.org/10.1002/jimd.12637. Distributed under Open Access license CC BY 4.0, without modification.
