Self-complementary AAV Vector Development Service
Recombinant adeno-associated virus (rAAV) has been successful in clinical trials for a variety of diseases. The self-complementary AAV (scAAV) is a recombinant viral vector engineered from a natural AAV and used as a tool for gene therapy. Creative Biolabs offers a comprehensive range of lab-made scAAV viruses with advantages including increased and extended transgene expression in vitro and in vivo, as well as higher in vivo DNA stability and more efficient cyclization.
Self-complementary AAV Vector Introduction
A key limitation of of conventional single stranded adeno-associated virus (ssAAV) vectors has been the nature of their genomic material, a single stranded DNA (ssDNA). In order to generate transgenic expression, the ssDNA genome must be converted to a transcriptionally active double stranded DNA (dsDNA) form. This rate limiting step is dependent on the host cell DNA polymerase mechanism and creates a significant time delay before gene expression can be initiated and also greatly reduces its overall efficiency. Thus, the development of scAAV vectors has become an exciting and revolutionary approach to overcoming this roadblock and to unlocking a new level of therapeutic potential.
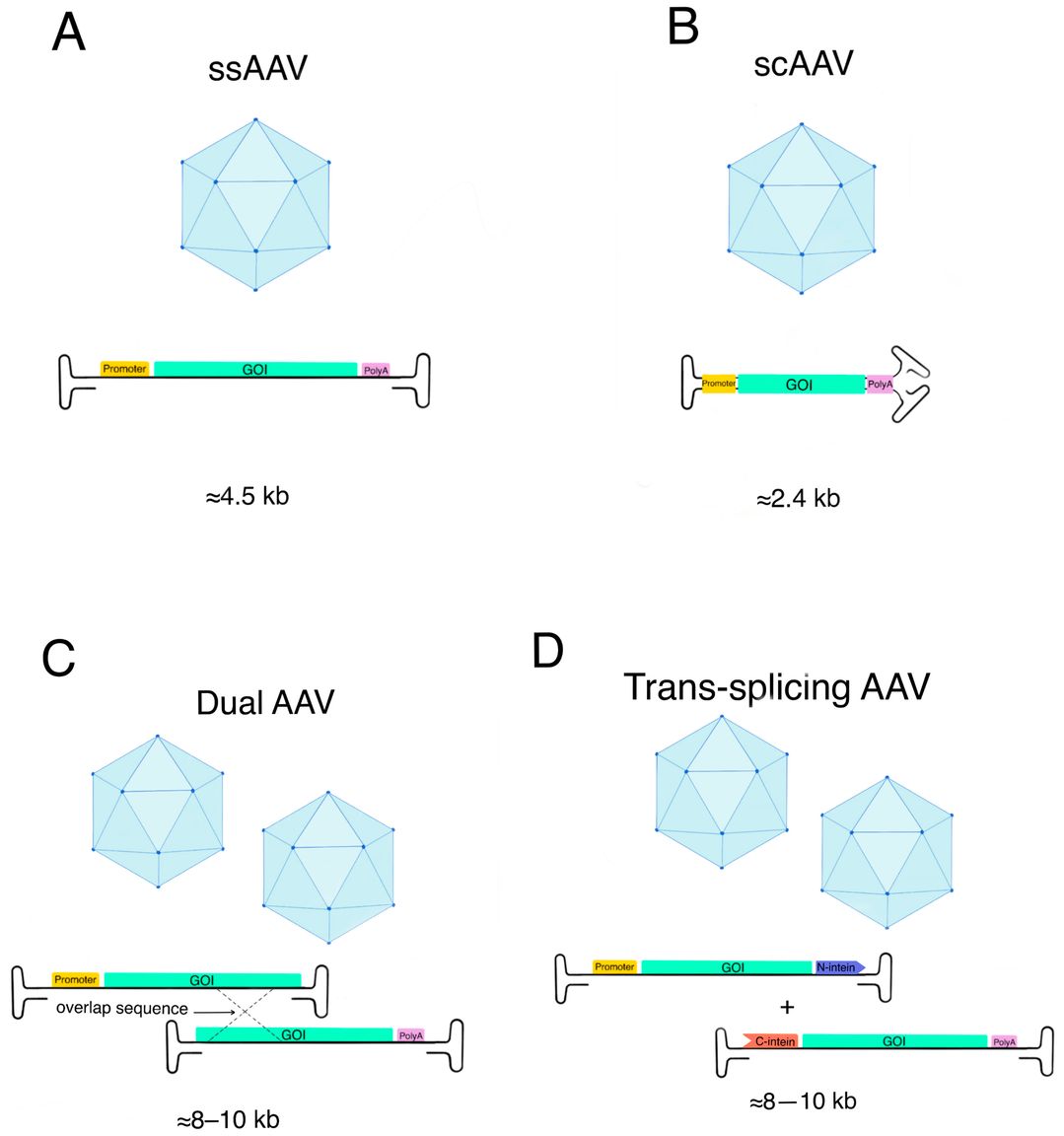 Figure 1. AAV vector strategy of delivery.1
Figure 1. AAV vector strategy of delivery.1
Self-complementary DNA AAV Vector
The unique structure of scAAV vector is the key to its outstanding performance. Unlike ssAAV vectors containing a single stranded DNA genome of approximately 4.7 kb, scAAV vectors contain a double stranded genome formed by annealing two complementary DNA strands. This is achieved by deleting an inverted terminal repeat (ITR) and inserting a complementary sequence, resulting in a self-complementary genome of approximately 2.4 kb (about half the size of ssAAV).
Self-complementary AAV Vector Manufacturing Methods
The production process of self-complementary adeno-associated virus (scAAV) vectors is similar to traditional single stranded AAV, but more refined. Its goal is to efficiently produce carriers with high titer and purity, ensuring the safety and effectiveness of downstream applications.
-
The Triple-Plasmid Transfection System
- ScAAV transfer plasmid: This plasmid is the core of the system. It contains a self-complementary gene box, which includes transgenes on both sides of the AAV reverse terminal repeat (ITR) sequence.
- Rep Cap plasmid: This plasmid contains AAV rep and cap genes.
- AAV helper plasmid (pHelper): This plasmid provides the genes required for helper viruses (usually adenoviruses).
-
Optimized Cell Culture and Transfection
The suspension culture system is scalable and provides a more controllable environment compared to traditional adherent culture. Our proprietary transfection protocol utilizes advanced polyethyleneimine (PEI) reagents, carefully designed to maximize plasmid uptake and subsequent viral production. This step is crucial as efficient transfection directly affects the final viral titer.
-
Downstream Processing and Purification
After viral particles are produced in production cells, they must be harvested and purified. Our robust downstream processing methods are designed to produce high-purity, high-titer products.
- Cell Lysis and harvest
- Nuclease treatment
- Purification
Self-Complementary AAV Vectors: Advances and Applications
The sc AAV vector represents a significant advancement in gene delivery technology. Unlike single stranded AAV (ssAAV), scAAV vectors are specially designed to package a genome composed of two complementary strands connected by a double stranded hairpin structure. This unique design allows the genome to immediately fold into double stranded DNA molecules upon entering the host cell nucleus, bypassing the speed limiting second strand synthesis step.
The rapid and stable transgenic expression of scAAV vector makes it particularly suitable for applications that require rapid onset of action. These applications include:
- Gene Replacement Therapy: used for genetic diseases that require rapid high-level protein expression to restore function.
- Genome Editing: scAAV vectors have shown excellent efficacy in delivering CRISPR-Cas9 components. Preclinical studies have shown that scAAV carrying guide RNA (gRNA) can significantly improve the efficiency of CRISPR-Cas9-mediated gene editing.
- Neurological Disorders: scAAV vectors are highly effective in the transduction of neurons and other non-dividing cells in the central nervous system (CNS), making them a powerful tool for gene therapy of diseases such as Huntington's disease or Rett syndrome.
Core Services at Creative Biolabs
At Creative Biolabs, we offer three categories of amino modifiers for our customers, respectively are 5' amino modifiers, 3' amino modifiers, and internal amino modifications. There are 8 types of 5'amino modifiers; 2 types of 3' amino modifiers; in terms of internal amino modifications, they refer to vary and/or altered dA, dC, dG and dT residues.
Vector Design and Construction Services
Our bioinformatics team provides advanced carrier design services that integrate the latest developments in the field of genetic element optimization. We offer:
- Customize promoter selection based on target tissue specificity and expression level requirements
- Codon optimization enhances transgenic expression
- Regulating component engineering, fine-tuning expression dynamics
- Report gene insertion, track transduction efficiency
scAAV Packaging and Production
We provide scalable scAAV production services, covering multiple capsid serotypes:
- Research grade production for preliminary in vitro and in vivo studies
- Preclinical grade carriers used for IND application research
- Clinical grade GMP production for human trials
- Batch production for commercial applications
Quality Control and Analysis
Our comprehensive quality control platform ensures the security, identity, purity, and efficacy of the carrier:
- Titer determination using digital PCR and ELISA detection
- Identity confirmation through next-generation sequencing
- Purity analysis, including evaluation of empty shells
- Using cell-based functional testing for efficacy determination
- Conduct sterile and endotoxin testing to meet regulatory standards
Reasons to Choose Creative Biolabs
- One-stop service: DNA synthesis, plasmid construction and preparation, scAAV packaging and purification
- High titer for animal injection
- Multiple AAV serotypes for transduction of various cell types
- Cost-effectiveness
- Customized solution
- Extremely effective
Frequently Asked Questions
Q: What are the main advantages of scAAV vector over ssAAV vector?
A: The main advantage is that transgenic expression is significantly faster and at a higher level. By packaging the double stranded genome, scAAV vectors bypass the rate limiting second strand DNA synthesis step required by ssAAV
Q: What is the packaging capacity of scAAV vector?
A: The packaging capacity of scAAV vector is about half of that of ssAAV vector, ranging from 2.2-2.4 kb, while ssAAV has a packaging capacity of 4.7 kb. This capacity reduction is due to the scAAV vector packaging two complementary chains of the transgenic expression cassette. Despite this size limitation, our optimized vector design allows for effective delivery of therapeutic genes through careful selection of compact regulatory elements and optimized coding sequences.
Q: How do you determine the appropriate virus titer for my experiment?
A: We recommend conducting dose-response experiments using a range of Infection Diversity Indicators (MOI) to determine the optimal titers for your specific application. Our technical support team can provide guidance based on your target cell type and experimental objectives. It is worth noting that transduction efficiency may vary depending on cell type, serotype, and experimental conditions, so empirical optimization is crucial for success.
Q: Which scAAV serotype should I choose for my project?
A: The choice of serotype depends on your target tissue. We offer natural serotypes (such as AAV2 for CNS/liver, AAV9 for heart/muscle, AAV8 for liver) and engineered serotypes with enhanced tropism. Our scientific team can assist you in selecting the optimal serotype based on your project objectives.
Q: Can you customize scAAV vectors according to my specific needs?
A: Yes, it is. We offer fully customizable carrier designs, including customized promoters, transgenes, capsids, and modifications (such as codon optimization, signal peptides). Our team works closely with you to design a vector that meets your project requirements.
Customer Review
"We will use Creative Biolabs' scAAV vector services for multiple customer projects, ranging from retinal malnutrition to cardiovascular disease. Their ability to customize carriers and deliver high-quality products on time makes them our preferred partner. The scientific support team is available to answer questions at any time, and their quality control data is reliable. Our clients are very satisfied with the results, and since partnering with Creative Biolabs, our preclinical research efficiency has significantly improved"
— Jennifer Rodriguez, Senior Scientist
Don't Hesitate, Contact Us!
The self-complementary AAV vector has become a powerful tool in gene therapy devices, overcoming the key limitations of single stranded vectors. Their ability to provide rapid and high-level transgenic expression paves the way for more effective and efficient treatment strategies. Creative Biolabs, with its profound expertise, comprehensive services, and commitment to quality, has a unique advantage in leveraging the full potential of scAAV vectors to advance your research and contribute to the future of disease research, making it a valuable partner for you. Please feel free to contact us for more details and our scientists will conduct further in-depth discussion on your project.
Reference
- Moldavskii D, Gilazieva Z, Fattakhova A, et al. AAV-Based Gene Therapy: Opportunities, Risks, and Scale-Up Strategies. International Journal of Molecular Sciences, 2025, 26(17): 8282. https://doi.org/10.3390/ijms26178282 Distributed under Open Access license CC BY 4.0, without modification.)
