AAV Vector Design Services for Central Nervous System (CNS) Disease
Introduction
Our AAV-based gene therapy services help overcome blood-brain barrier delivery challenges for neurodegenerative and monogenic diseases, aiding in developing effective CNS therapies via advanced vector engineering and targeted delivery. Creative Biolabs offers comprehensive AAV expertise, providing a seamless path from concept to therapeutic candidate, with specialized vector engineering for optimal CNS biodistribution and transduction efficiency.
Central Nervous System Disease Gene Therapy
The CNS, protected by the blood-brain barrier (BBB), is hard to treat for neurodegenerative and monogenic diseases. Recent advances in gene therapy, especially AAV vectors, are changing this. AAVs are non-pathogenic, low-immunogenic, and engineerable to deliver therapeutic genes to the brain and spinal cord, promising to treat once-incurable conditions.
Central Nervous System Disease
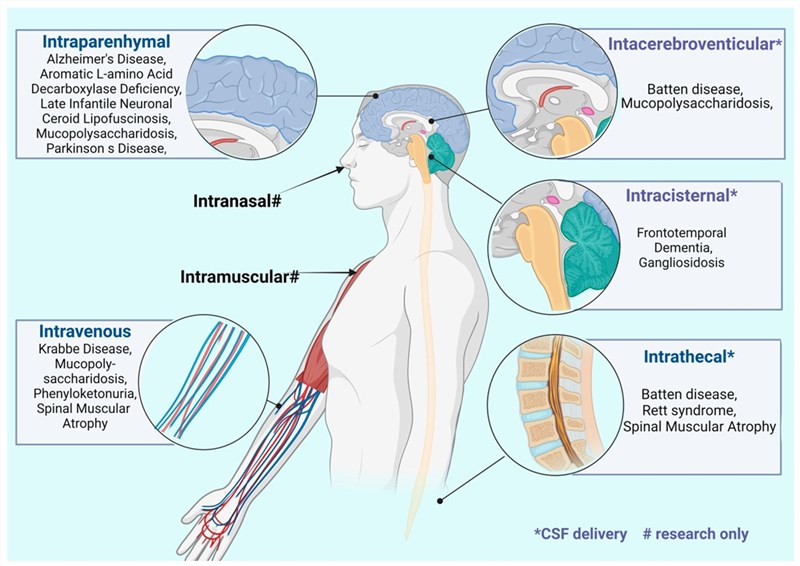 Fig.1 Common central nervous system diseases and the administration methods when using rAAV for corresponding treatments.1
Fig.1 Common central nervous system diseases and the administration methods when using rAAV for corresponding treatments.1
Diseases of the central nervous system (CNS) refer to a broad category of disorders affecting the brain, spinal cord, and associated peripheral neural tissues. Their etiologies are complex, encompassing genetics, infection, trauma, degeneration, metabolic abnormalities, and other factors. These conditions often cause neurological impairment, severely affecting patients' motor, cognitive, and sensory functions.
Common Types and Typical Diseases
-
Neurodegenerative diseases
Characterized by progressive degeneration and loss of neurons, these diseases typically have a chronic, progressive course and poor prognosis.- Alzheimer's disease (AD): The most common form of dementia, caused by β-amyloid deposition and abnormal phosphorylation of tau protein, leading to a gradual decline in cognitive functions (memory, thinking, judgment).
- Parkinson's disease (PD): Degeneration of dopaminergic neurons in the substantia nigra, manifesting as motor dysfunction such as resting tremor, bradykinesia, and muscle rigidity.
- Huntington's disease (HD): An autosomal dominant genetic disorder caused by mutations in the Huntingtin gene, resulting in damage to basal ganglia neurons, characterized by involuntary choreiform movements, cognitive decline, and psychiatric symptoms.
-
Monogenic diseases
Caused by mutations in a single gene, these diseases often onset in childhood or adolescence, with distinct symptoms directly linked to specific genetic defects.- Spinal muscular atrophy (SMA): Caused by mutations in the SMN1 gene, leading to the death of motor neurons in the anterior horn of the spinal cord, manifesting as muscle weakness, atrophy, and in severe cases, life-threatening respiratory dysfunction.
Core Difficulties in Treatment
- Limitation of the BBB: As a CNS-protective "natural barrier," the BBB blocks harmful substances but also hinders most drugs (e.g., macromolecular therapeutics, gene therapy vectors) from penetrating, impeding effective delivery to lesions.
- Non-regenerability of nerve cells: Mature neurons have minimal regenerative capacity. Severe damage (e.g., neuronal loss in degenerative diseases) makes function hard to restore, requiring reliance on replacement therapies or protection of remaining neurons.
- Complexity of etiology: Most CNS diseases (e.g., AD, PD) have incompletely understood etiologies, often involving multiple mechanisms (genetics, environment, immunity, etc.), limiting the efficacy of single-target treatments.
- Immunological and safety risks: The CNS has a unique immune microenvironment. Therapeutic interventions may trigger immune responses, affecting efficacy and potentially causing side effects like neuroinflammation.
Creative Biolabs offers a wide range of recombinant AAV products and provides customized services for gene therapy of a series of central nervous system diseases, mainly listed as follows:
Workflow
We provide a clear and systematic approach to gene therapy development, ensuring transparency and quality at every step.
- Required Starting Materials: To initiate a project, we require foundational information such as your target gene of interest, the specific CNS disease you are addressing, and any existing preliminary data on potential vector serotypes or animal models.
- Vector Design & Serotype Selection: We select/engineer suitable AAV serotypes (e.g., AAV9, AAV-rh.10) based on target cells and delivery routes, design expression cassettes with optimized promoters/therapeutic genes, yielding a vector blueprint.
- Vector Production & Purification: Produce high-titer, clinical-grade AAV via proprietary platforms, with multi-stage purification to ensure high purity (minimal empty capsids/contaminants), resulting in ready-to-use vectors.
- In Vitro & In Vivo Validation: Rigorous testing: in vitro assays confirm gene expression in neuronal/glial cells; in vivo studies in animal models evaluate biodistribution, long-term expression, and therapeutic effect, providing proof-of-concept data.
- Immunogenicity Assessment: Analyze immune response potential (e.g., neutralizing antibodies) to ensure patient safety and clinical translatability, generating a clear immunogenicity profile.
- CMC & Regulatory Support: Provide comprehensive CMC documentation/support for regulatory filings, ensuring compliance with IND submission standards, delivering a complete regulatory data package.
- Final Deliverables: Upon project completion, you will receive a comprehensive technical report detailing all experimental procedures and results, purified AAV vector lots with accompanying quality control data, and a summary of key findings and recommendations for future steps.
- Estimated Timeframe: The typical timeframe for a full-service project range from 4 to 12 weeks, depending on the complexity of the target disease and the scope of required in vivo studies.
Discover How We Can Help - Request a Consultation
What We Can Offer
At Creative Biolabs, we are committed to providing a comprehensive and customizable suite of services to accelerate your CNS gene therapy project. As experts in the field, we understand that each research endeavor has unique requirements, and our offerings are designed to provide the flexibility and scientific rigor you need.
Customized Vector Design & Optimization
We engineer AAV vectors for optimal tropism, high expression, and minimal immunogenicity, including codon optimization and promoter selection to maximize transgene expression in target cells.
GMP-Certified Vector Production
Our GMP-compliant facility uses strict aseptic procedures, ensuring consistent quality and smooth transition from research to clinical development.
One-Stop Service
End-to-end support from concept to validated candidate, covering vector design, production, and in vitro/in vivo validation, saving time and resources.
Large-Scale Production Capabilities
Advanced capabilities meet preclinical and clinical demands, ensuring a stable, reliable supply of high-quality vectors for large-scale projects.
Advanced Quality Control
Our system uses PAT and QbD, with high-standard QC for every batch to guarantee purity, titer, and potency.
Regulatory & IP Support
We provide comprehensive documentation and support to navigate intellectual property and regulatory submissions, backed by regulatory expertise.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
What are the main challenges in gene therapy for CNS diseases?
The primary challenge is bypassing the blood-brain barrier to deliver therapeutic genes effectively. Additionally, pre-existing neutralizing antibodies in patients can hinder treatment success. Our AAV vector engineering and selection strategies are specifically designed to address these challenges, ensuring optimal delivery and reduced immunogenicity.
How do you ensure the quality of the AAV vectors?
We maintain a rigorous quality control process, including comprehensive testing for vector purity, titer, and potency. Our manufacturing process minimizes empty capsids and aggregates, ensuring you receive a high-quality product that meets the stringent requirements for preclinical and clinical studies.
Do you offer services for custom AAV vector design?
Yes, in addition to our established serotypes, we specialize in custom vector engineering. Whether you need a specific capsid modification to enhance tropism or a unique expression cassette for your gene of interest, our team can design and produce a vector tailored to your precise research needs.
Should you be prepared to advance your CNS gene therapy project, our proficient team stands ready to deliver the necessary scientific guidance and technical solutions. We encourage you to engage with us to elaborate on your specific requirements and gain deeper insights into how our services can expedite the progression of your research.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Słyk, Żaneta, Natalia Stachowiak, and Maciej Małecki. "Recombinant Adeno-Associated Virus vectors for Gene Therapy of the Central Nervous System: delivery routes and clinical aspects." Biomedicines 12.7 (2024): 1523. https://doi.org/10.3390/biomedicines12071523. Distributed under Open Access license CC BY 4.0, without modification.
