Active Targeting with Proteins and Antibodies
Active targeting with proteins and antibodies enables receptor-guided transport, resulting in higher cellular uptake and more targeted exposure than passive EPR alone. In the review, Creative Biolabs will provide a practical playbook—comparing ADCs with ligand-decorated carriers, outlining core conjugation methods, and establishing design/assay benchmarks—to enable scalable, on-target delivery.
Introduction to Active Targeting with Proteins and Antibodies
What Is Ligand-Based Active Targeting?
Drug delivery via traditional chemotherapy is largely non-specific, where cytotoxic agents are unable to target diseased cancer cells alone, causing damage to normal healthy cells as well. This limitation of traditional chemotherapy has led to the development of the field of nanomedicine, which aims to improve specificity. One key strategy is the use of "active targeting". Unlike passive targeting, which relies on the enhanced permeability and retention (EPR) effect of tumor blood vessels to trap nanoparticles, active targeting takes a more precise approach: it uses biologically active molecules (ligands) conjugated to nanocarriers (such as magnetic nanoparticles, or MNPs) to bind directly to specific receptors that are overexpressed on cancer cells (Figure 1). In simple terms, active targeting is like putting a "GPS tag" on your drug. Given the nine global approvals and an extensive late-stage ADC drug pipeline that have been disclosed, ligand-based active targeting has firmly secured its position in real-world clinical settings.
At Creative Biolabs, we offer a range of consulting solutions and services specifically designed for targeted drug delivery, thereby enhancing the success of your experiments.
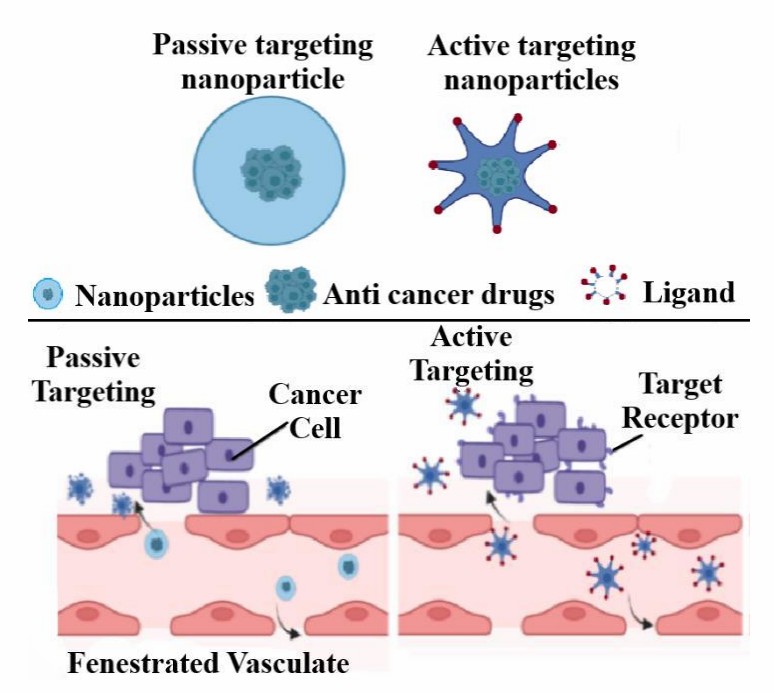 Fig.
1 The comparison between the passive and active targeting.1
Fig.
1 The comparison between the passive and active targeting.1
Why Proteins & Antibodies Make Strong Ligands
Proteins and antibodies are among the most effective ligands for active targeting. Antibodies, for example, can be designed to recognize specific antigens present on the surfaces of tumor cells (such as HER2 in breast cancer), which allows nanocarriers loaded with chemotherapeutics, photothermal agents, or imaging probes to be guided to cancer cells exclusively. Proteins, such as folate, can also function by binding to receptors that are required for the growth of cancer cells, thus further enhancing targeting specificity. This precision not only maximizes drug concentration at the tumor site but also minimizes off-target toxicity.
As research in nanomedicine advances, active targeting with proteins and antibodies has become a cornerstone of smart drug delivery systems, especially when paired with magnetic nanoparticles (MNPs). For example, paclitaxel-loaded Fe3O4 magnetic nanoparticles conjugated with anti-HER2+ antibodies achieved superior antitumor efficacy compared to non-targeted carriers by leveraging external magnetic fields to guide carriers toward HER2+ tumors while using ligand-receptor binding to ensure final, cell-specific delivery. By combining various targeting strategies, cancer therapies can be made more personalized and less invasive.
In summary, four points make proteins and antibodies potent ligands:
- High specificity: Antibodies recognize precise epitopes, which improves selectivity and reduces off-cell binding.
- Nanomolar affinities: Many therapeutic antibodies bind in the low-nanomolar range, which supports robust target engagement even at modest ligand densities.
- Built-in internalization: Many receptors internalize after the antibody binds, which is ideal for endocytic delivery or ADC payload release.
- Format flexibility: You can utilize whole IgG for avidity and half-life, or use fragments (Fab/scFv) for smaller size and less steric hindrance on crowded carrier surfaces.
Target Selection Framework (Scorecard You Can Use Today)
Before you decide what kind of ligands you want to use, you need to decide "which receptor" first. Here, you can structure the decision using a short scorecard and assign a weight of 1–5 to each criterion for comparison.
Criteria to score
- Disease-biased expression (breadth, heterogeneity across cohorts)
- Internalization rate and recycling behavior
- Receptor density on the target cells of interest
- Safety window and off-tissue expression
- Translational toolbox (availability of validated antibodies, assays, animal models)
Example (illustrative only)
Table 1 Evaluation of disease-related receptors.
| Receptor | Disease bias | Internalization | Density | Safety | Toolbox | Total |
|---|---|---|---|---|---|---|
| HER2 | 5 | 4 | 5 | 3 | 5 | 22 |
| TfR (BBB) | 4 | 5 | 4 | 3 | 5 | 21 |
| CD98hc (BBB) | 4 | 5 | 4 | 3 | 4 | 20 |
According to Table 1, it is clear that each receptor is suited to different application use cases based on its strengths. HER2's main strengths are disease specificity and target density, making it suitable for use cases that require high priority on those factors, such as cancer therapies that require precise tumor targeting. The transferrin receptor (TfR) stands out for blood-brain barrier (BBB) drug delivery, as it balances strong internalization (key to crossing the BBB) with a comprehensive translational toolbox. Meanwhile, CD98 heavy chain (CD98hc) (BBB) functions as a viable alternative to TfR for BBB applications but requires additional investment to expand its translational toolbox—specifically by validating more antibodies or animal models—to match TfR's utility.
Delivery Formats & Conjugation Strategy
To achieve active targeting, choosing the proper delivery format is critical. There are two main categories for active targeting with antibodies and proteins: antibody-drug conjugates (ADCs) and ligand-decorated carriers (Figure 2).
ADCs
ADCs integrate a monoclonal antibody with a payload via a defined linker and drug-to-antibody ratio (DAR). They can deliver significant specificity without the assistance of a nanocarrier. By exploiting "cleavable" (e.g., enzyme-, acid-, or reduction-sensitive) and "non-cleavable" linkers, scientists can strike a balance between the circulatory stability and intracellular release of he therapeutic drugs. Compared to random lysine coupling, site-specific conjugation exhibits tighter control over heterogeneity and improved developability, supporting more efficient biotherapeutic development.
Ligand-decorated carriers
Ligand-decorated carriers (e.g., immunoliposomes, ligand-NPs) offer payload flexibility. Their surfaces are "addressed" with antibodies or proteins to trigger receptor-mediated uptake. However, antibody orientation and site-specific conjugation are crucial for preserving binding after attachment. Poor orientation can lead to reduced affinity and uptake. To control orientation, engineered handles and enzymatic methods should be applied so that the antigen-binding site can face outwards. In this way, both function and batch consistency could be preserved.
For more information related to Polymeric Nanoparticle- and Lipid Nannoparticle-based Delivery Strategies, you could visit the Resource Pages:
Polymeric Nanoparticle;Lipid Nannoparticle
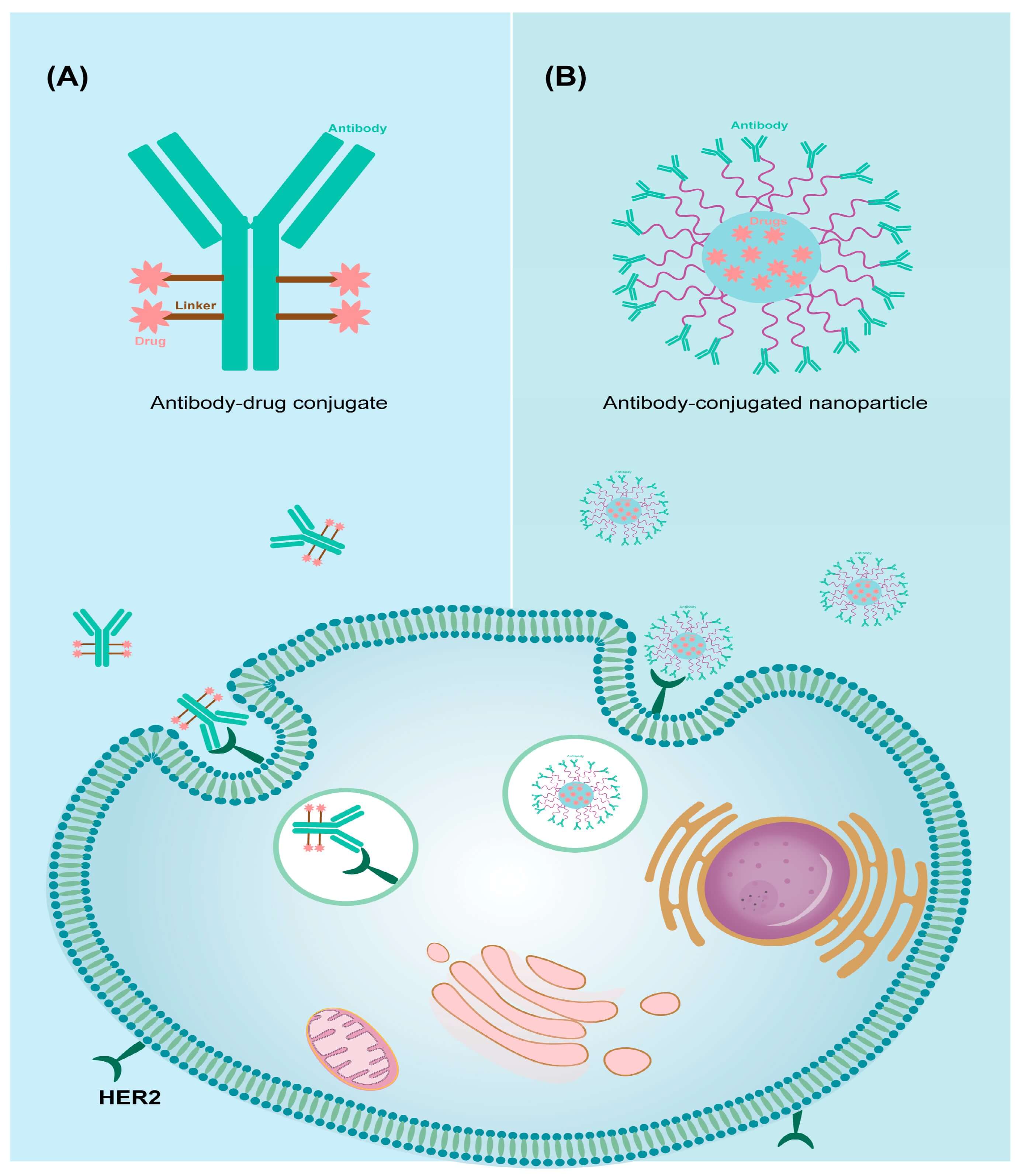 Fig.
2 Antibody-mediated targeted drug delivery systems. (A) ADCs. (B) Antibody-conjugated nanoparticles.2
Fig.
2 Antibody-mediated targeted drug delivery systems. (A) ADCs. (B) Antibody-conjugated nanoparticles.2
Design Knobs That Drive Uptake
Affinity (Kd) & avidity—engineer for productive binding, not just tight binding.
Higher affinity improves initial capture, but ultra-tight binding can cause "rim-sticking" (surface residency without endocytosis). Therefore, the best result often comes from the right affinity class and multivalency. It is essential to strike a balance between Kon and Koff that supports receptor clustering and internalization within the receptor's recycling window.
Ligand density—map the sweet spot, don't guess.
As too few ligands lead to weak binding and too many lead to increased corona formation or blocked endocytosis, it is important to explore the density zone that allows for targeting.
Too few ligands weaken capture, while excess display amplifies the protein corona, creates steric crowding, and can block endocytosis. Therefore, it is necessary to tune the number of ligands per particle and their spacing to identify the density that maximizes uptake on a per-dose basis.
Particle size & surface—control fate before you control binding.
Size and surface chemistry dictate circulation half-life, biodistribution, and the usable "real estate" for ligand presentation. Mid-nano diameters with near-neutral or mildly negative ζ-potential and low-fouling shells (PEG/zwitterionic) are generally selected as they typically reduce opsonization. Incorporation of smart coatings (charge-shifting, pH-responsive) can further boost cellular entry.
Internalization rate—choose receptors that traffic your payload.
Rapid endocytosis is essential for delivering payloads—especially endosome-activated molecules—whereas slow, surface-resident receptors waste dose and invite clearance. Therefore, receptors that internalize rapidly and recycle compatibly (e.g., TfR, EGFR, integrins) should be selected, and internalization half-times and dominant pathways (clathrin or caveolae) should be measured to inform design. Quantitative internalization and co-localization assays should be utilized to confirm trafficking kinetics and pathways, ensuring activation occurs in the intended intracellular compartment.
Analytics & Release Testing: Proving It's "Active"
To separate true active targeting from non-specific sticking, a QC checklist should be created for every batch. The checking points should include:
- Antigen binding preserved after conjugation (ELISA/BLI/SPR);
- Orientation confirmation (site-specific tags or Fc-based reads);
- Internalization assay with your target cells;
- Selectivity vs isotype control and receptor-block;
- Stability (linker/Fc), sterility, endotoxin, and bioburden.
Related Services You May Be Interested in
FAQs
What is the difference between passive and active targeting in drug delivery?
Passive targeting depends on size and blood flow to accumulate in tissues. Active targeting adds a binding step using proteins or antibodies, which can raise cell-level uptake via receptor-mediated endocytosis.
Why use antibodies as ligands for targeted delivery?
Antibodies give high specificity and low-nanomolar binding that supports selective uptake. They often trigger internalization after binding, which helps bring the payload into the cell.
Which receptors are most used for antibody/protein-guided delivery?
In oncology, HER2, TROP2, BCMA, CD22, CD33 and others are common. For brain delivery across the BBB, TfR and CD98hc are leading shuttle targets with distinct biodistribution profiles.
How common is HER2 positivity in breast cancer?
According to guideline documents and reviews, about 15-20% HER2 positivity are among invasive breast cancers.
What design parameters matter most for on-target uptake?
Affinity/avidity, ligand density, particle size/surface, receptor density, and internalization kinetics.
How many ADCs are approved, and what does that signal?
There are 19 global ADC approvals as of June 2025, and 24 more candidates in Phase III. This validates antibody-guided delivery at scale.
Commitment: How Creative Biolabs Accelerates Your Program
At Creative Biolabs, we support your ligand-based targeting from idea to in vivo data. The services we provide include target and ligand strategy, antibody and protein engineering, and site-specific conjugation.
References
- Spoială, A. et al. "Smart Magnetic Drug Delivery Systems for the Treatment of Cancer." Nanomaterials 13, 876 (2023). https://www.mdpi.com/2079-4991/13/5/876 Distributed under Open Access license CC BY 4.0, without modification.
- Yan, S., Na, J., Liu, X. & Wu, P. "Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy." Pharmaceutics 16, 248 (2024). https://www.mdpi.com/1999-4923/16/2/248 Distributed under Open Access license CC BY 4.0, without modification.
- Tang, Z. et al. "Overcoming the On‐Target Toxicity in Antibody‐Mediated Therapies via an Indirect Active Targeting Strategy." Advanced Science 10, 2206912 (2023). https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202206912
- Shi, P. et al. "Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics." J Nanobiotechnol 21, 103 (2023). https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-023-01826-1
- Chen, M. Z. et al. "A versatile antibody capture system drives specific in vivo delivery of mRNA-loaded lipid nanoparticles." Nat. Nanotechnol. 20, 1273–1284 (2025). https://www.nature.com/articles/s41565-025-01954-9



