Vitamins in Drug Delivery: Mechanisms andPlatforms
Vitamins are no longer just nutrients — they're becoming intelligent tools in modern drug delivery design. By functioning as bioactive ligands, stabilizers, and permeability enhancers, vitamins enable drugs to reach their targets more precisely and stay effective longer. This dual biological and formulation role is driving new strategies in nanomedicine, from vitamin-decorated nanoparticles to co-delivery systems that pair vitamins with chemotherapeutics. At Creative Biolabs, we explore how vitamin-based delivery platforms can bridge the gap between biological selectivity and pharmaceutical efficiency, advancing the next generation of targeted therapeutics.
Introduction to vitamins in drug delivery
Why Vitamins Matter in Drug Delivery
Vitamins, long recognized as essential nutrients for human physiology, have emerged as transformative tools in drug delivery due to their unique biological properties and safety profiles. Their relevance in this field stems from two core advantages: innate biological compatibility and targeted transport mechanisms. In contrast to synthetic carriers, vitamins (such as B12, D3) are recognized by the body's uptake mechanisms (vitamin B12 binding to transcobalamin (TC) and receptor CD320; vitamin D binding to vitamin D-binding protein (DBP) and vitamin D receptor (VDR)), providing them with an "off-ramp" to biological barriers (gastrointestinal tract, blood-brain barrier, etc. ), minimizing off-target toxicity while improving the bioavailability of poorly soluble or unstable therapeutics. Additionally, vitamins address key challenges in drug delivery: they protect payloads from degradation (e.g., B12 shields antibiotics from gastric enzymes) and enable controlled release, which is critical for chronic treatments such as cancer or neurological disorders.
What Are Vitamin-Based Drug Delivery Systems?
By leveraging the unique biophysical properties of vitamins, vitamin-based drug delivery systems integrate vitamins as targeting ligands, carriers, or encapsulated payloads. Vitamin-based drug delivery systems use vitamins in three ways:
Vitamin-decorated carriers
A nanoparticle, liposome, micelle, or nanoemulsion is capped with a vitamin ligand (for example, B12) that binds a receptor and triggers cell entry.
Vitamin–drug conjugates or prodrugs
A vitamin is chemically linked to a drug to improve uptake, solubility, or tissue distribution.
Vitamin-enabled excipient systems
Some vitamins, such as Vitamin E TPGS, can work as excipients that increase solubility, reduce efflux, or enhance permeability.
When to choose a vitamin ligand vs. a vitamin excipient
- if you need cell-type targeting or receptor-mediated uptake. → Use a ligand
- if your primary problem is poor solubility, permeability, or efflux. → Use an excipient
At Creative Biolabs, we integrate vitamin ligands into modular, targeted delivery systems to help sponsors move from idea to data with fewer iterations.
- See our Module Delivery Systems for ligand selection, conjugation, and platform options.
Mechanisms: How Vitamins Improve Targeting and Exposure
Vitamins improve delivery through well-defined biological and physicochemical mechanisms:
Receptor-mediated internalization
Vitamin B12 (B12) excels in targeted delivery due to its highly specific receptor-driven uptake system. Naturally, B12 binds to transcobalamin (TC) in the bloodstream, forming a B12-TC complex that is actively internalized by cells via the CD320 receptor—a glycoprotein overexpressed in rapidly proliferating cells (e.g., gastric cancer, melanoma, breast cancer cells) and neurons (Figure 1). This trait can be harnessed by decorating drug carriers with B12. For example, B12-conjugated sterically stabilized liposomes show enhanced uptake by B16F10 melanoma cells and greater tumor accumulation in mice, while B12-functionalized diatom microalgae target CD320-overexpressing HT-29 colorectal cancer cells. Beyond cancer, B12 can also be used to improve oral delivery. By leveraging the gastrointestinal tract's intrinsic absorption machinery (binding to intrinsic factor in the duodenum, followed by the cubam receptor in the ileum), B12 can enhance the oral bioavailability of conjugated therapeutics, such as antibiotics or peptides.
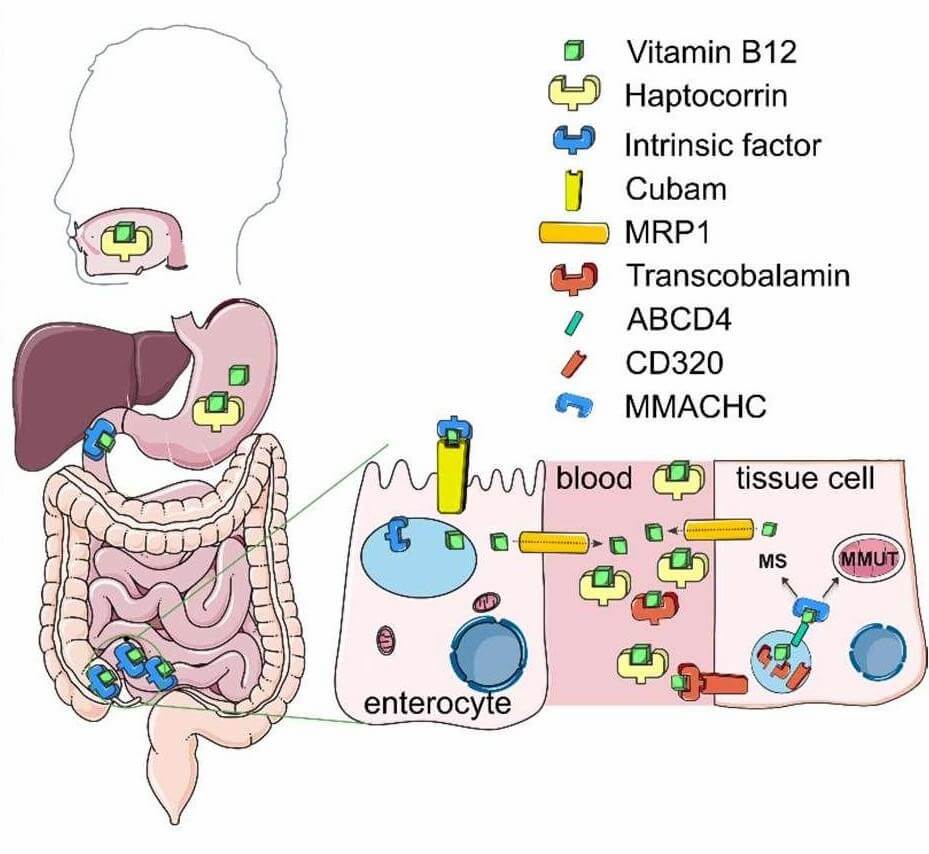 Fig.1
In vivo transport of B12.2
Fig.1
In vivo transport of B12.2
Transport and absorption
Fat-soluble vitamins such as vitamin D (D3) can enhance systemic exposure by leveraging lymphatic transport to bypass the hepatic portal circulation, thus avoiding the first-pass metabolism. Conventional oral drugs often undergo rapid liver degradation; however, vitamin D-loaded carriers, such as nanostructured lipid carriers (NLCs) and nanoemulsions, prioritize lymphatic uptake, thereby minimizing liver-mediated breakdown. A typical example for this is the in vivo studies of the vitamin D-loaded NLCs, which employs lymphatic transport and exhibits higher serum 25(OH)D3 levels (the active circulating form of vitamin D) compared to free vitamin D. This mechanism is critical for chronic therapies (e.g., vitamin D supplementation for osteoporosis or immune disorders) where sustained systemic levels are essential.
Permeation and efflux modulation
Vitamins address two key barriers to drug exposure: poor membrane permeability and efflux pump-mediated drug exclusion. For example, in Caco-2 cell monolayer studies, conjugation of vitamin B12 to therapeutics (e.g., the antibiotic colistin) could increase intestinal permeability. Additionally, vitamin E-TPGS can inhibit P-glycoprotein (P-gp), an efflux pump that expels drugs. Moreover, vitamin B12 can indirectly support permeation by stabilizing drug carriers (e.g., liposomes) in biological fluids, ensuring payloads reach target cells instead of being degraded or excluded.
Co-signaling/co-therapy
Vitamins can act as "bioactive carriers" that not only deliver therapeutics but also modulate biological pathways to enhance treatment effects. For instance, vitamin D can bind to the vitamin D receptor (VDR) to regulate immune responses and cancer cell differentiation, thus sensitizing tumors to chemotherapy. Therefore, by combining with 1,25(OH)2D3 (the active form of vitamin D), vitamin B12 can boost anticancer activity in HeLa and MCF-7 cells via caspase-4/8-mediated apoptosis, without harming normal cells. Moreover, in co-delivery systems (e.g., B12 + cisplatin-loaded chitosan nanoparticles), vitamin D can reduce chemotherapy doses by sensitizing tumors, minimizing systemic toxicity while maintaining efficacy.
Platforms: From Lipids to Polymers to Emulsions
The existing vitamin-based drug delivery systems are based on several different platforms. The decision about which platform to use for particular vitamin formulations depends mainly on their physicochemical characteristics, together with the desired therapeutic effect. Lipid-based, polymeric, and emulsion systems have emerged as the forerunners in this field (Table 1). As can be observed from Table 1, polymeric nanoparticles can provide a controlled release and a stable loading. They also allow clean surface chemistry for vitamin ligands, including spacer engineering to tune binding and internalization. Lipid systems such as liposomes, solid lipid nanoparticles, and LNPs offer strong compatibility with lipophilic vitamins and hydrophobic drugs. They are a natural fit for Vitamin E and K components, and can be tuned for size, charge, and release. Micelles and nanoemulsions assemble quickly and can deliver hydrophobic molecules through oral, dermal, or parenteral routes. Using TPGS micelles offers dual benefits: solubilization and modulation of P-glycoprotein (P-gp).
Table 1 Comparison of different vitamin delivery platforms.
| Platform Type | Core Advantages | Suitable Vitamin Types | Typical Application Scenarios |
|---|---|---|---|
| Polymeric Nanoparticles | 1. Excellent controlled release for long-acting administration; | Water-soluble vitamins (e.g., Vitamin B12)、Water-soluble vitamin derivatives (e.g., folate-biotin conjugates) | 1. Vitamin B12-conjugated PLGA-PEG nanoparticles: Target CD320-overexpressing gastric cancer cells to enhance tumor accumulation of chemotherapeutics (e.g., cisplatin). |
| 2. Controllable surface chemistry for easy vitamin ligand modification; | 2. Folate-biotin-pullulan nanoparticles: Load doxorubicin for dual-targeting via folate receptors and SMVT receptors, boosting cytotoxicity against breast cancer. | ||
| 3. High payload stability to protect cargoes from degradation | 3. Biotin-modified chitosan nanoparticles: Load 5-fluorouracil to target ASGPR receptors on hepatoma cells, reducing toxicity to normal liver tissue. | ||
| Lipid Systems | 1. High compatibility with lipophilic vitamins and hydrophobic drugs; | Lipophilic vitamins (e.g., Vitamin D3, Vitamin E) | 1. Vitamin D3-loaded nanostructured lipid carriers (NLCs): Chitosan coating improves stability; oral administration reduces first-pass metabolism via lymphatic transport, increasing serum 25(OH)D3 levels. |
| 2. Good biosafety and easy metabolism in the human body; | 2. Vitamin B12-functionalized liposomes: Modified with transferrin to enhance blood-brain barrier penetration for Alzheimer's therapy. | ||
| 3. Tunable size, charge, and release behavior | 3. Vitamin E-loaded liposomes: Load curcumin to enhance drug retention in the skin stratum corneum via Vitamin E's membrane penetration effect. | ||
| Micelles/Nanoemulsions | 1. Rapid self-assembly and simple preparation process; | Lipophilic vitamins (e.g., Vitamin E, Vitamin D3)、Water-soluble vitamins (e.g., Vitamin B12) | 1. Vitamin E-derived TPGS micelles: Load paclitaxel to solubilize the drug and inhibit P-glycoprotein (P-gp), reversing tumor multidrug resistance. |
| 2. Multi-route administration (oral, dermal, parenteral); | 2. Vitamin B12-loaded nanoemulsions: Sunflower oil as oil phase and Tween 80 as surfactant, enhancing B12 permeability in the intestine. | ||
| 3. Dual functions of solubilization and functional regulation | 3. Vitamin D3-loaded nanoemulsions: Added with Tween 80 and soy lecithin to improve stability in dairy products for food fortification. |
Design knobs to consider
Key Design Knobs include ligand density (e.g., 0.36 µmol/g folate + 0.67 µmol/g biotin for maximal uptake), particle size (50–150 nm for EPR effect synergy), ζ potential (neutral/weakly negative for colloidal stability), and triggered release (pH-sensitive linkers for endosomal escape, redox-responsive bonds for tumor microenvironments).
For a flexible mix-and-match approach to lipid, polymer, and hybrid platforms (e.g., lipid-polymer hybrid nanoparticles for vitamin D), explore our Module Delivery Systems.
Therapeutic Applications
Oncology
Vitamin ligands can lead to higher tumor uptake and lower doses, which is enhanced in combination therapies with chemotherapies. In a preclinical study, Vitamin D3 and paclitaxel (PTX) polymeric nanoparticles demonstrated stronger tumor control with reduced systemic toxicity. The PEG spacers minimized opsonization while vitamin D3 synergized with PTX to reduce the systemic toxicity. This setting may account for the increasing attention to vitamin D's anticancer properties through the VDR-mediated activation and PLGA-based co-delivery strategies.
Metabolic and endocrine
In metabolic and endocrine disorders, where chronic management and stable therapeutic levels are critical, vitamin-decorated carriers address key challenges of oral delivery for fragile molecules, while enabling sustained release to boost patient adherence and reduce dose-related peaks and troughs. An example is B12-decorated PEG-PLGA NPs, which utilize CD320 receptor-mediated endocytosis (the standard mechanism of B12 transport) to enhance the oral bioavailability of labile drug molecules. Careful tuning of the B12 ligand density on the nanoparticle surface is crucial to avoid steric crowding that would negatively impact receptor binding and thus internalization. As a result, significantly higher AUC and Cmax values were observed compared to the non-decorated PEG-PLGA control, directly correlating with improved payload bioavailability.
The sustained drug release from these vitamin-decorated carriers can further mitigate fluctuations in blood concentration, thereby reducing dose peaks/troughs and improving patient adherence. This stability is particularly valuable for chronic metabolic conditions (e.g., B12 deficiency-related metabolic disturbances), as it reduces the frequency of administration and supports long-term patient adherence.
Deficiency therapy
Long-acting depot formulations, parenteral formats for malabsorption, and stabilized oral systems can upgrade standard vitamin therapies by improving exposure and convenience. For example, Vitamin E-derived TPGS micelles can overcome solubility/efflux limits of hydrophobic active pharmaceutical ingredients (APIs) by boosting solubility, reducing P-gp-mediated efflux (validated in Caco-2 models), and delivering a cleaner PK profile.
Emerging areas
Recent advancements include CNS-accessible B12-decorated nanoparticles and topical Vitamin D/E applications for skin disorder treatment, amongst other innovative vitamin-based drug delivery systems. Clearly, vitamin-based DDSs have shifted away from conventional supplementation strategies to a more targeted and precision-based therapy approach.
Challenges and Solutions
Although Vitamin-based drug delivery systems offer unique advantages, such as targeted transport and enhanced bioavailability, multiple challenges, including vitamin instability and biological heterogeneity, pose significant obstacles to the practical use of vitamin-driven drug delivery systems in clinical settings.
Instability of vitamins (D, E, K)
As oxidation and light can degrade potency, antioxidants, oxygen-barrier packaging, and amber containers are recommended for use. The integrity of vitamins should be regularly validated under ICH conditions.
Efflux and first-pass effects
Even with better solubility, efflux may limit exposure. TPGS systems and lymphatic-first routes can help. Consider food-effect studies for oral designs.
Scale-up and reproducibility
- Keep process parameters tight.
- Use in-line/at-line analytics where possible.
- For large programs, a continuous manufacturing assessment is required.
Biological heterogeneity
As receptor density varies, preclinical design should include biomarker plans and enrichment strategies to avoid false negatives.
How Creative Biolabs Helps
We provide an integrated path from ligand selection to analytics:
Scope we cover: vitamin ligand screening (B12/D/E/K), conjugation chemistry (click, EDC/NHS, maleimide), carrier selection (lipid, polymer, hybrid), in vitro ADME/transport, release and permeability studies, stability and photostability, and preliminary in vivo models when appropriate.
Workflow:
- Design sprint: target profile, ligand choice, platform shortlist, and assay map.
- Bench build: rapid prototypes across 2-3 platforms; early stability and release.
- Down-selection: Select the best formulation based on pre-set CQAs.
- Deep characterization: orthogonal analytics, mechanism confirmation, and dose-range checks.
- Scale & transfer: process window, control strategy, and documentation.
Deliverables: complete formulation dossier, raw data, method SOPs, CoA-style analytics, and a scale-up plan.
Explore our Targeting Module Development Services to plug services into your existing workflow.
Related Services You May Be Interested in
FAQs
What role do vitamins play in drug delivery?
Vitamins can guide carriers to receptors, boost membrane transport, and stabilize hard-to-formulate drugs. Therefore, they enable targeted uptake, better bioavailability, and lower doses. In some designs, vitamins also serve as co-therapies, which can enhance response while maintaining toxicity in check.
Which vitamins are most used as ligands or enhancers?
Vitamin B12 is popular for receptor-mediated uptake. Vitamin D appears in co-therapy and signaling designs. Vitamin E–TPGS helps with solubility and reduces P-gp efflux. Vitamin K can support lipid systems and stability. The best choice depends on target tissue, route, and API properties.
Do vitamin-decorated nanoparticles improve oral delivery?
Yes, they can. By binding to intestinal receptors and improving permeability, vitamin-decorated systems increase exposure to drugs that usually have weak oral absorption. Yet, success depends on ligand density, particle size, release behavior, and GI stability under fed and fasted states.
How do Vitamin E–TPGS systems help with poorly soluble drugs?
TPGS forms micelles that solubilize hydrophobic APIs and reduce P-gp efflux. This dual effect increases apparent permeability and can smooth the pharmacokinetic profile. However, the formulation must balance the TPGS concentration to achieve micelle stability, viscosity, and, if applicable, injectability.
Conclusion
Vitamins provide drug delivery with three powerful levers: precise targeting, enhanced bioavailability, and practical stability. When you combine these with the right platform, you can lower dose, improve safety, and open routes that once seemed out of reach. If your program requires faster iteration with precise analytics, our team can help you select the right vitamin ligand or excipient and develop a formulation that withstands real-world constraints.
Ready to design your vitamin-based drug delivery system?
Talk with our scientists and launch a two-week design sprint that delivers prototypes, data, and a clear next step. Explore our Targeting Module Development Services and contact us today.
References
- Aggeletopoulou, I., Kalafateli, M., Geramoutsos, G. & Triantos, C. "Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery." Biomolecules 14, 1090 (2024). https://www.mdpi.com/2218-273X/14/9/1090.
- Kuldyushev, N. A. et al. "From Nutrient to Nanocarrier: The Multifaceted Role of Vitamin B12 in Drug Delivery." IJMS 26, 5119 (2025). https://www.mdpi.com/1422-0067/26/11/5119 . Distributed under Open Access license CC BY 4.0, without modification.
- Jurczyk, M. et al. "Single- versus Dual-Targeted Nanoparticles with Folic Acid and Biotin for Anticancer Drug Delivery." Pharmaceutics 13, 326 (2021). https://www.mdpi.com/1999-4923/13/3/326.



