AAV Vector Design Services for Gene Therapy
Introduction
Our AAV Vector Design for Gene Therapy service accelerates gene therapy programs via advanced protein engineering and high-throughput screening, delivering high-quality vectors for precise therapeutic delivery. Creative Biolabs addresses wild-type AAV limitations, offering comprehensive design, optimization, and production. Clients get custom vectors with enhanced tropism, reduced immunogenicity, and optimal transgene expression, supporting research and clinical trials.
AAV Vector Design for Gene Therapy
Adeno-associated virus (AAV) is a leading in vivo gene delivery tool, valued for non-pathogenicity, durable expression without host genome integration. Our expertise is applied to a wide range of therapeutic areas, tailoring AAV vectors to meet the unique challenges of each disease.
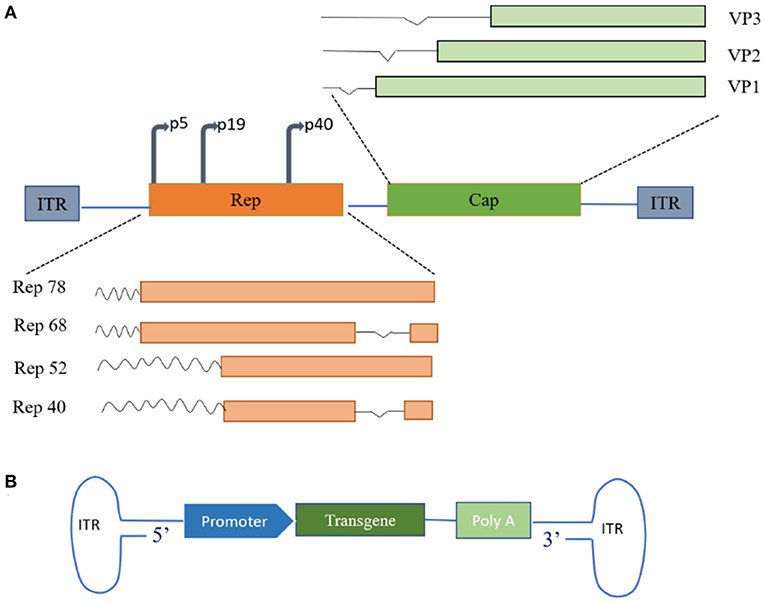 Fig.1 Schematic diagrams of the genomic structures of wild-type and recombinant AAV. Wild-type AAV mainly includes replication genes (Rep) and capsid genes (Cap), while recombinant AAV includes therapeutic transgenes, reverse terminal repeat sequences, and capsid proteins.1
Fig.1 Schematic diagrams of the genomic structures of wild-type and recombinant AAV. Wild-type AAV mainly includes replication genes (Rep) and capsid genes (Cap), while recombinant AAV includes therapeutic transgenes, reverse terminal repeat sequences, and capsid proteins.1
Central Nervous System Disease
AAVs (especially AAV9) can cross the blood-brain barrier. We engineer vectors to enhance this ability, targeting neurons and glial cells for conditions like SMA, Batten disease, and Parkinson's. Our capsids improve CNS penetrance, enabling systemic delivery to broad brain/spinal cord regions without invasive injection.
Liver Directed Disease
The liver is ideal for AAV delivery, key for inherited metabolic disorders like hemophilia A/B. Our vectors are designed for high-efficiency, long-term protein expression in hepatocytes. Optimized liver tropism achieves high expression at lower doses, reducing off-target effects and costs.
Ocular Disease
Direct intraocular AAV injection treats inherited blindness and retinal diseases. We optimize vectors for precise delivery to photoreceptors and retinal pigment epithelium. Leveraging the eye's immune privilege, our vectors maximize transduction in these cells, offering potential one-time treatments.
Other Hereditary Disease
Our platform adapts to genetic disorders affecting muscle, heart, inner ear, etc., via tropism-tailored vectors. For Duchenne muscular dystrophy (large gene delivery), we use dual-vector systems. Tissue-specific promoters ensure therapeutic protein expression only in target tissues, minimizing side effects.
Workflow
-
Step 1: Rational Design & In Silico Modeling
We use advanced computational tools and machine learning to model capsid variants, predicting amino acid mutations that enhance tropism and avoid immune epitopes, yielding promising candidates for testing. -
Step 2: Directed Evolution Library Construction
Based on computational analysis, we built a diverse AAV capsid variant library for rapid screening, resulting in a physical library ready for high-throughput screening. -
Step 3: High-Throughput Screening & Selection
Our platforms screen the library to identify variants with superior transduction efficiency and target cell specificity, quickly pinpointing top candidates. -
Step 4: Lead Candidate Validation & Optimization
Top candidates undergo rigorous in vitro/in vivo validation (expression levels, immunogenicity), producing a fully characterized lead vector. -
Step 5: Process Development & Scale-Up
We develop a robust, scalable manufacturing process for the lead vector, enabling consistent scaling from research to clinical-grade batches, with a reproducible protocol.
Upon completion, you will receive a final report detailing all design and validation data, a vial of your custom-engineered AAV vector, and a comprehensive quality control data package. The typical timeframe for this service ranges from 8 to 12 weeks, depending on the complexity of the target tissue and the scope of the project.
Discover How We Can Help - Request a Consultation
What We Can Offer
As a professional in the biological sciences, you are well aware that gene therapy necessitates a personalized approach rather than a universal solution. At Creative Biolabs, we take pride in delivering customized, integrated strategies that align with the distinct requirements of your specific project. We function not merely as a product provider but as a collaborative partner, offering full-lifecycle support to facilitate the success of your program.
Customized Design and Optimization
We offer bespoke AAV vector design, leveraging our expertise in advanced protein engineering to meet your specific needs for enhanced tropism and gene expression.
High-Throughput Screening Platforms
Our state-of-the-art platforms allow for the efficient screening of millions of capsid variants to identify the most potent and selective candidates for your therapeutic application.
Rigorous Quality Assurance
Documentation of strain origin and stability is a core part of our service, assessed and approved by our qualified quality assurance team. We ensure the stability of your vectors in cell banks and throughout large-scale production.
GMP-Certified Production
We adhere to the highest standards of Good Manufacturing Practice (GMP), with strict aseptic verification procedures throughout the entire production process to guarantee the quality and safety of every vector.
Flexible Fermentation Modes
We can optimize culture conditions and run fermentation processes in batch, fed-batch, or continuous modes to maximize yield and efficiency based on your specific project requirements.
Comprehensive Quality Control
We use high-standard quality control tools to quantify and evaluate the quality of our AAV vectors, providing you with the reliable data you need for regulatory filings and clinical success.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
How do you address the issue of pre-existing immunity to AAV?
Our capsid engineering platform is designed to identify and engineer novel AAV variants that are less susceptible to neutralization by pre-existing antibodies. This approach helps ensure that your therapy can be effective in a broader patient population.
Can you help with packaging a large gene that exceeds the standard AAV capacity?
Yes, we specialize in advanced solutions for payload size challenges. We utilize innovative strategies such as dual-vector systems to deliver genes larger than the native AAV capacity, ensuring your therapeutic program is not limited by size constraints.
How do your AAV vectors compare to other viral or non-viral delivery methods?
Our engineered AAV vectors offer superior transduction efficiency and provide durable, stable gene expression. We avoid the safety concerns associated with viral integration and the lower efficiency of many non-viral alternatives, making AAV the gold standard for many in vivo applications.
Our team of experts is dedicated to helping you accelerate your gene therapy program. We offer a comprehensive partnership from initial design to clinical-grade manufacturing. We are confident that our expertise and cutting-edge technology can provide the solutions you need to bring a life-changing cure to patients.
Contact Our Team for More Information and to Discuss Your Project
Reference
- Zhang, Huili, et al. "AAV-mediated gene therapy: Advancing cardiovascular disease treatment." Frontiers in cardiovascular medicine 9 (2022): 952755. https://doi.org/10.3389/fcvm.2022.952755. Distributed under Open Access license CC BY 4.0, without modification.
