AAV Vector Design Services for Liver Directed Disease
Introduction
Facing long development cycles, ineffective in vivo delivery, or poor sustained therapeutic expression for genetic liver disorders, our custom rAAV design service accelerates research and enables durable, high-quality gene expression via innovative protein engineering and advanced recombinant DNA technology. It offers comprehensive solutions for inherited liver disease gene therapies, providing project-tailored deliverables (optimized vectors, high-titer particles, validation data) and addressing issues like pre-existing AAV immunity to boost clinical success.
Discover How We Can Help - Request a Consultation
Liver Directed Disease
The liver is a prime target for gene therapy due to its unique anatomical features, high metabolic activity, and the large number of genetic diseases that affect its function. Gene therapy aims to introduce a functional copy of a defective gene into hepatocytes using viral vectors like recombinant adeno-associated viruses (rAAV). This approach offers a curative treatment by restoring normal protein function. This includes several diseases, such as:
Pompe Disease
A rare genetic disorder caused by a deficiency of the acid alpha-glucosidase (GAA) enzyme, leading to glycogen accumulation in cells, particularly in the liver and muscles.
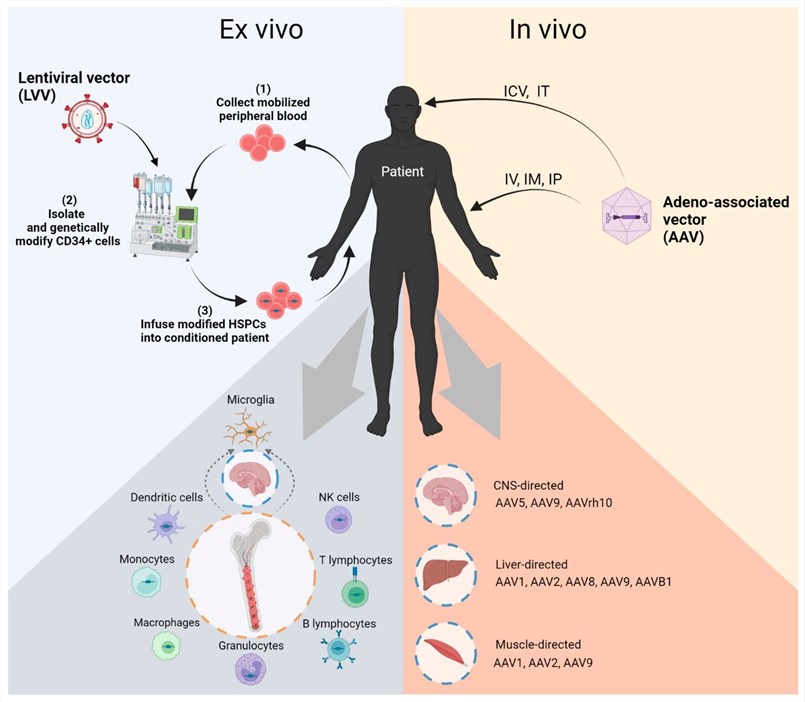 Fig.1 In vivo AAV gene therapy for Pompe disease. By using different AAV vector capsid proteins and administration routes, target different tissue microenvironments.1,3
Fig.1 In vivo AAV gene therapy for Pompe disease. By using different AAV vector capsid proteins and administration routes, target different tissue microenvironments.1,3
Alpha-1 Antitrypsin Deficiency
A genetic disorder where the liver doesn't produce enough alpha-1 antitrypsin protein, which protects the lungs from damage. This can lead to severe liver and lung disease.
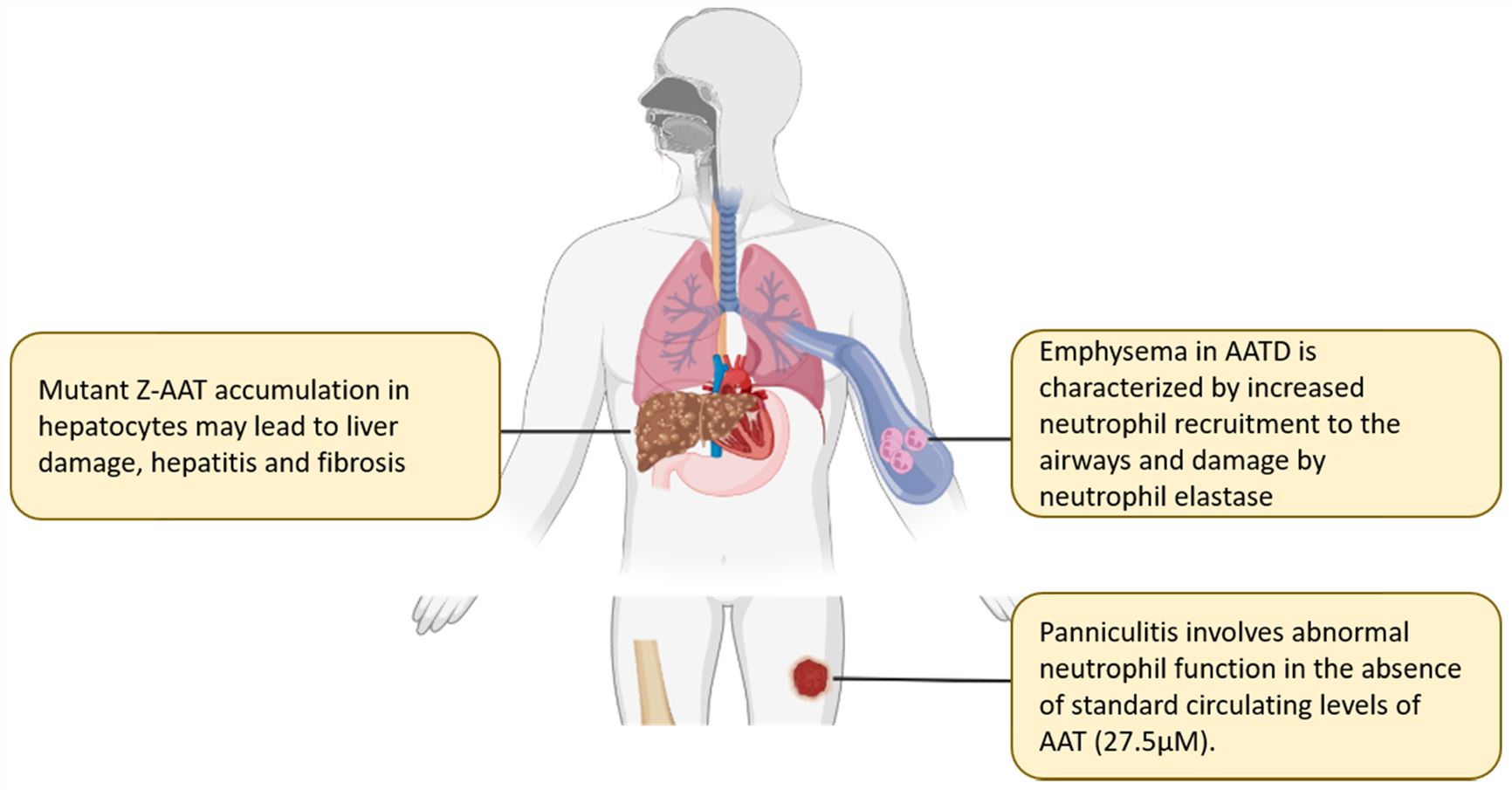 Fig.2 The clinical significance of α-1 antitrypsin deficiency.2,3
Fig.2 The clinical significance of α-1 antitrypsin deficiency.2,3
Hemophilia A/B
Inherited bleeding disorders caused by a deficiency of clotting factors VIII and IX, respectively. The liver is the primary site of production for these factors, making it an ideal target for gene therapy.
Workflow
- Required Starting Materials: To begin, we need a clear project description outlining your research goals and target disease. Key materials include the sequence of your gene of interest (cDNA), details on the required promoter and any regulatory elements, and information on the target disease.
- Vector Design and Optimization: Design/optimize rAAV vectors for liver tropism—select suitable serotypes, design stable high-expression promoters, and add regulatory sequences. Outcome: Custom rAAV vector blueprint.
- Gene Synthesis and Plasmid Construction: Synthesize target genes and ligate them into optimized rAAV backbones for accurate therapeutic construct assembly.
- High-Titer rAAV Production: Use advanced cell culture and triple-plasmid transfection to produce scalable, clinical-grade high-titer particles for preclinical/clinical studies. Outcome: Large quantities of purified vectors.
- Purification and Quality Control: Purify via advanced chromatography; conduct rigorous QC to verify titer, purity, and integrity for safe, effective products.
- In Vitro and In Vivo Validation (Optional): Perform in vitro transduction assays and optional in vivo animal studies to confirm vector functionality, tropism, efficacy, and safety.
- Final Deliverables: You will receive a comprehensive project report detailing the entire process, including sequence verification data, quality control reports (titer, purity, integrity), and the final high-titer rAAV product.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 12 weeks, depending on the complexity of the vector design and the scope of the project. Factors such as the number of constructs and the inclusion of validation studies can influence the duration.
What We Can Offer
At Creative Biolabs, we are committed to providing a comprehensive, customized rAAV solution for your liver-directed gene therapy research. Our advantages include:
One-stop, End-to-End Service
We provide a seamless workflow from initial vector design and optimization through to final high-titer rAAV particle delivery.
Customized Service
Our services are fully customizable to meet your specific research goals, including tailored vector design, promoter selection, and transgene optimization.
Scalable Production
Our scalable production methods, including batch and fed-batch fermentation, can meet the demands of both preclinical and large-scale clinical trials.
Strict Quality Assurance
We adhere to a robust Quality-by-Design (QbD) framework and strict aseptic procedures, ensuring the highest standards of product consistency, purity, and safety.
In-house Expertise
Our team provides comprehensive support and guidance, including codon optimization for maximum expression and advice on managing host immune responses.
Rigorous Quality Control
We utilize high-standard quality control tools and comprehensive documentation to guarantee vector quality and stability.
Experience the Creative Biolabs Advantage - Get a Quote Today
Customer Reviews

FAQs
What types of genetic diseases can be targeted with your liver-directed rAAV service?
Our service is designed to address a wide range of monogenic liver diseases, including but not limited to Alpha-1 Antitrypsin Deficiency, Pompe disease, and Hemophilia A/B. We also have experience with other metabolic disorders that manifest in the liver. We encourage you to reach out to our team to discuss your specific target disease and project goals.
How do you ensure the long-term expression of the therapeutic gene?
We employ several strategies to ensure durable expression, including the selection of liver-specific and robust promoters, as well as the optimization of the rAAV vector genome. The non-integrating nature of rAAV vectors in non-dividing hepatocytes allows for stable episomal expression, which is a key advantage for long-term gene therapy.
What if my target gene is too large to fit in an AAV vector?
The AAV vector has a packaging capacity limit of approximately 4.7 kilobases (kb). If your gene of interest exceeds this size, we can propose and implement advanced strategies, such as dual-vector systems or mini-gene approaches, to ensure successful delivery. Please contact our team to explore these options in more detail.
Creative Biolabs is committed to providing best-in-class rAAV solutions for your gene therapy research. Our expertise in Liver Directed Disease and our comprehensive service workflow will help you achieve your project milestones efficiently and effectively.
Contact Our Team for More Information and to Discuss Your Project
References
- Unnisa, Zeenath, et al. "Gene therapy developments for Pompe disease." Biomedicines 10.2 (2022): 302. https://doi.org/10.3390/biomedicines10020302.
- O'Brien, Michael E., et al. "A review of alpha-1 antitrypsin binding partners for immune regulation and potential therapeutic application." International journal of molecular sciences 23.5 (2022): 2441. https://doi.org/10.3390/ijms23052441.
- Distributed under Open Access license CC BY 4.0, without modification.
