Antisense Oligonucleotide (ASO) Design and Synthesis Service
To assist customers quickly and efficiently enter the primary screening phase with antisense oligonucleotide (ASO) candidate sequences, Creative Biolabs offers specialized custom ASO drug sequence design and synthesis services. Upon receiving the transcription sequence/ID number of the target sequence, species information (human, murine, rattus, non-human primates (NHP), etc.), and the target modification type, Creative Biolabs can provide different design schemes and a variety of control options such as positive control, negative control, scramble control, etc.
Antisense Oligonucleotide (ASO)
ASO is a single-stranded oligonucleotide molecule (18~30nt), which can be a heterozygous single strand of DNA, RNA or DNA/RNA. After entering cells, ASO can lead to mRNA degradation under the action of RNase H1 by complementing the target sequence, thus inhibiting protein expression; or through steric effect to achieve the selective splicing of pre-mRNA, regulate the translation of genes, to achieve the purpose of disease treatment.
Antisense Oligonucleotide Delivery
The therapeutic efficacy of any antisense oligonucleotide (ASO) is closely related to its ability to reach its intracellular site of action. Naked oligonucleotides face numerous challenges, including rapid degradation by nucleases in serum, filtration by the kidneys, and the inability to passively cross cell membranes due to their polyanionic nature. Strategic chemical modification of the oligonucleotide backbone and glycosyl groups is a primary approach to improving their stability and pharmacokinetics. Furthermore, overcoming delivery barriers often requires advanced formulation strategies.
- Systemic Delivery
- Local and CNS Delivery
Antisense Oligonucleotide Toxicity
Sequence-independent toxicity is often related to the overall structure of the molecule, including modifications to the phosphate thioester (PS) backbone, which are crucial for nuclease resistance. While PS modifications are essential for stability, they can also lead to nonspecific protein binding, thereby activating complement, promoting platelet aggregation, and accumulating in tissues such as the liver and kidneys.
Antisense Oligonucleotide (ASO) Therapy
This fundamental technology has a wide range of applications, enabling various therapeutic modalities beyond simple mRNA degradation. This versatility is key to addressing a variety of diseases.
- Splicing Regulation - Oligonucleotides can be programmed to bind to the splicing site of precursor mRNA, forcing the cellular splicing mechanism to skip an exon (exon skipping) or include a cryptic exon (exon inclusion).
- Transcription Stabilization and Translational Arrest - Certain chemical modifications can create steric hindrance, allowing oligonucleotides to physically block ribosomes or spliceosomes without triggering RNase H.
- Targeting Non-coding RNA (ncRNA) - Oligonucleotides designed to inhibit specific miRNAs (often called antimiRs or blockmirs) can relieve the miRNA-mediated natural translational repression, thereby upregulating the expression of the target protein.
Oligonucleotide Chemical Modification and Design Technology
The therapeutic index of oligonucleotides is directly related to their chemical structure, which determines their stability, binding affinity, and cellular uptake. Creative Biolabs employs a generational chemical modification approach to optimize these key parameters.
First Generation
Introducing phosphate thioester (PS) backbone substitution, where non-bridging oxygen atoms are replaced by sulfur atoms. This modification significantly enhances resistance to endogenous nucleases and prolongs the molecule's half-life in vivo. The PS backbone is the basic structure of almost all clinically available constructs.
Second Generation
These modifications primarily occur at the 2' position of the ribose. Key second-generation modifying groups include 2'-O-methyl (2'-OMe) and 2'-O-methoxyethyl (2'-MOE). These modifications increase binding affinity to target RNA, enhance nuclease resistance, and generally reduce off-target toxicity.
Third Generation
This type of nucleic acid includes locked nucleic acids (LNA) and their analogues. The principle is to link the 2'-oxygen atom and the 4'-carbon atom of the ribose ring, thereby "locking" the sugar conformation in the C3'-endoform conformation of the RNA mimicking.
Antisense Oligonucleotides Primers Design
For researchers conducting antisense oligonucleotide (ASO) projects, several fundamental decisions must be made:
- Chemical backbone: The choice of chemical groups (e.g., phosphate thioester backbone, 2'-O-methoxyethyl, bridging nucleic acids such as LNA or BNA) will determine its pharmacokinetic and pharmacodynamic properties.
- Species-specific design: Sequences used for in vitro human cell studies and in vivo mouse models require different design parameters to account for variations in sequence differences and off-target effects.
Antisense Oligonucleotide (ASO) Design and Synthesis Services
ASO Design
Our team of bioinformaticians and molecular biologists utilizes a suite of computational tools to generate a library of candidate antisense oligonucleotides (ASOs) targeting your specific needs. We provide a detailed report explaining the rationale behind each candidate ASO, including predicted targeting efficiency and off-target effect scores.
ASO Synthesis
We produce high-purity ASOs using state-of-the-art solid-phase synthesis. Our chemical expertise encompasses a wide range of modification and coupling chemistry (e.g., GalNAc, fluorescent dyes, cholesterol). Each batch undergoes rigorous quality control, including analytical high-performance liquid chromatography (HPLC) for purity assessment and mass spectrometry for identification.
ASO Chemical Modification
Creative Biolabs can provide custom designed ASOs with modified phosphate skeleton, base, and sugar ring, so as to improve their stability, binding potency and affinity, and to reduce off-target effect, dose and frequency of administration. At present, the commonly used modification on the market is 5-10-5 2 '-MOE gapmer substitution modification, which supports more than 200 modifications such as LNA, thioate, methylation, 2' -O-Me, etc. Customers can submit specific requirements according to the anticipated effects of the ASO drugs.
Tab.1 Features of modified
| Type | Sites | Nuclease resistance | Affinity | RNase H | Other Features |
|---|---|---|---|---|---|
| PO ASO | —— | × | |||
| PS ASO | Phosphate Skeleton | ↓ | √ | Shorter half life | |
| 2'-MOE | Sugar | √ | ↓ | √ | Gapmer |
| 2'-F | Sugar | √ | × | Uniform modification | |
| LNA | Sugar | √ | √ | √ | Gapmer |
| Cholesterol/Fatty acids | Conjugation | Better targeting and uptake |
Highlights of ASO Design
- Provide professional ASO sequence design, comprehensively consider ASO length, GC content, thermodynamic characteristics, specificity, chemical modification and other characteristics;
- Customers can choose different modified bases, purification methods, and synthesis scale to meet the specific experimental needs;
- Supports high-throughput design, ASOs can be designed for multiple transcripts, cross-species design;
- Design according to various mechanisms of action (based on steric hindrance formation mechanism or mRNA degradation mechanism);
- Improved intracellular stability and reduced toxicity through nuclease-resistant modification and flexible chimeric design.
When designing ASO sequence, we mainly refer to the following design steps:
 Figure 2 ASO sequence design and screening.
Figure 2 ASO sequence design and screening.
The selection of a screening ASO sequence is a balance of several factors, with different priorities depending on the intended application of the ASO. Screening is mainly to remove nucleic acid sequences that may cause inflammation or other adverse reactions, such as CpG. If cross-species ASOs sequences are intentionally selected, the selection factors depend on the degree of sequence homology and the importance of cross-species homology to the project.
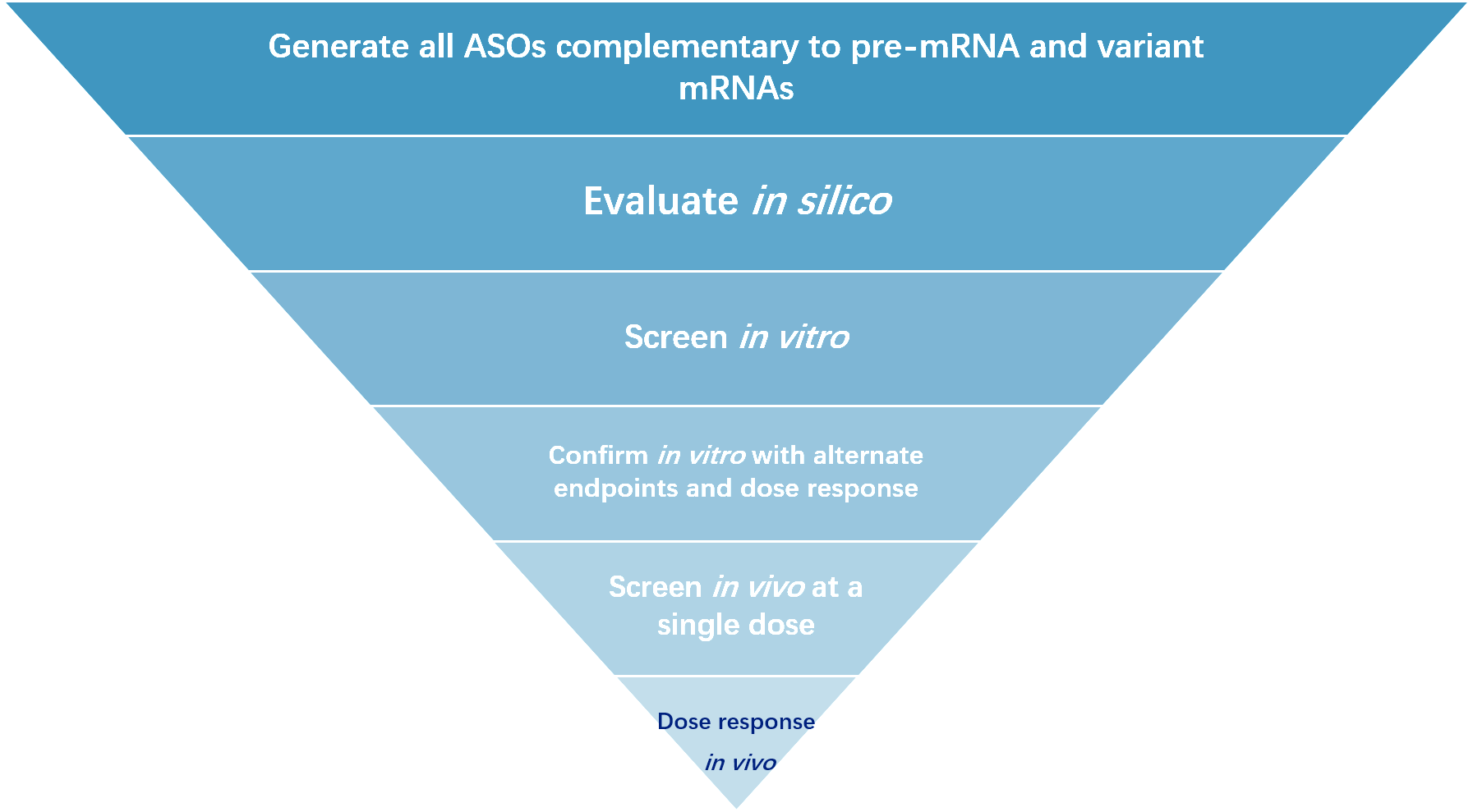 Figure 3 Basic process of ASO selection.
Figure 3 Basic process of ASO selection.
Our Collaborative Process
- Consultation: We begin by understanding your target, goals, and specific requirements.
- Proposal: We provide a detailed project plan, timeline, and quote.
- Design & Synthesis: Our team executes the bioinformatic design and synthesizes the ASO panel.
- Validation & Analysis: We perform the agreed-upon in vitro and/or in vivo studies.
- Reporting & Support: You receive a comprehensive data package and our scientists are available for detailed discussion.
Frequently Asked Questions
Q: What is the minimum scale you can provide for initial screening?
A: We typically provide nanomolar-level synthesis, which is ideal for high-throughput in vitro screening and early cell experiments. Our synthetic capabilities are highly flexible, scaling from approximately 50 nanomolars to several grams.
Q: How do you ensure the specificity of my ASO and avoid off-target effects?
A: We employ a multi-step bioinformatics screening process targeting the entire transcriptome and recommend empirical validation in relevant cell models to confirm targeting activity and rule out significant off-target effects.
Q: Can you synthesize ASOs for animal models?
A: Absolutely. We can design specific sequences for mice, rats, primates, and other common model organisms, and have extensive experience in formulating ASOs suitable for various routes of administration (in vivo jetPEI, invivofectamine, saline).
Q: What purity can I expect?
A: Our standard products have a purity ≥90% (as determined by HPLC). Higher purity grades (≥95%, ≥98%) are available upon request.
Q: Do you offer gapmer structure design services?
A: Yes. Gapmer design is one of our core strengths. It comprises a central deoxyribonucleotide region flanked by chemically modified flanks (e.g., 2'-MOE or LNA-type modifications). Our designed structures optimize RNase H cleavage efficiency while ensuring excellent stability and affinity.
Q: How do you protect my intellectual property?
A: All projects are bound by strict confidentiality and non-disclosure agreements (CDA/NDA). Our internal data management system employs a segmented security design to ensure the strictest confidentiality of your sequence data and project details. From initial consultation to final delivery, we always prioritize intellectual property protection.
Connect with Us Anytime!
At Creative Biolabs, we leverage world-class expertise to help you translate gene targets into highly effective, high-quality therapeutic candidates. We are ready to support your most ambitious projects, from initial lead compound discovery to preclinical manufacturing, helping you accelerate the clinical application of next-generation gene therapies. Contact us today to discuss how our advanced platform solutions can help you unlock the potential of the next biomedical breakthrough.
Reference
- Di Fusco D, Dinallo V, Marafini I, et al. Antisense oligonucleotide: basic concepts and therapeutic application in inflammatory bowel disease. Frontiers in pharmacology, 2019, 10: 305. https://doi.org/10.3389/fphar.2019.00305 (Distributed under Open Access license CC BY 4.0, without modification.)
