In Vivo Study Service for Antisense Therapeutics
In the preclinical research phase of antisense oligonucleotides (ASO), animal metabolism, efficacy, safety, and pharmacokinetics studies are required. The data obtained from in vivo studies can provide important references for clinical trial design, so as to determine the appropriate dose, frequency, and route of administration, and ensure the effectiveness and safety of clinical trials. Creative Biolabs offers a variety of animal disease models and reference ASO drugs to meet the research needs of customers.
Antisense Therapeutics
Antisense oligonucleotides (ASOs) have evolved from niche experimental tools into a validated third pillar of drug discovery, standing alongside small molecules and biologics. By interacting directly with RNA, ASOs offer the unique ability to target the "undruggable genome"—proteins that lack enzymatic active sites or accessible pockets—thereby opening vast new frontiers in clinical application. The application landscape of antisense therapeutics has expanded dramatically, driven by advances in chemical stabilization and targeted delivery:
Neurological & Neuromuscular Disorders
The Central Nervous System (CNS) remains the most successful arena for ASOs. Because ASOs can be delivered intrathecally to achieve high local concentrations with long half-lives, they are revolutionizing the treatment of neurodegenerative diseases.
Cardiovascular & Metabolic Diseases
The advent of N-acetylgalactosamine conjugation has transformed ASOs into potent therapies for liver-associated targets. This technology allows for subcutaneous administration with high hepatic uptake, significantly lowering systemic toxicity.
Oncology
Cancer cells often rely on transcription factors and signaling proteins that are notoriously difficult to target with small molecule inhibitors. ASOs bypass protein structure entirely by degrading the coding mRNA, effectively "cutting the supply line" of oncoproteins.
Rare Genetic & Infectious Diseases
ASOs are inherently programmable. This allows for rapid development cycles for ultra-rare mutations. Additionally, in the realm of infectious disease, ASOs are being developed to target conserved viral RNA sequences, aiming for functional cures by preventing viral replication.
Animal Models for In Vivo Studies
Tumor Models
ASO has shown remarkable ability and potential in anti-tumor, providing a new choice and hope for tumor therapy. In order to verify its metabolic characteristics, gene silencing efficiency, and safety in vivo, it is necessary to conduct studies in corresponding animal models.
- Renal cell carcinoma model: By constructing a mouse model of renal cell carcinoma, ASO drug therapy by intratumoral injection or tail vein injection can successfully inhibit the growth of renal cell carcinoma in vivo.
- Breast cancer model: In a mouse model of breast cancer, estrogen-induced tumor growth was significantly reduced by intratumoral or intravenous ASO.
- Hepatocellular carcinoma model: The mouse model of hepatocellular carcinoma offers several advantages, including high genetic homology to humans, low cost, robust reproducibility, and effective simulation of human disease pathology.
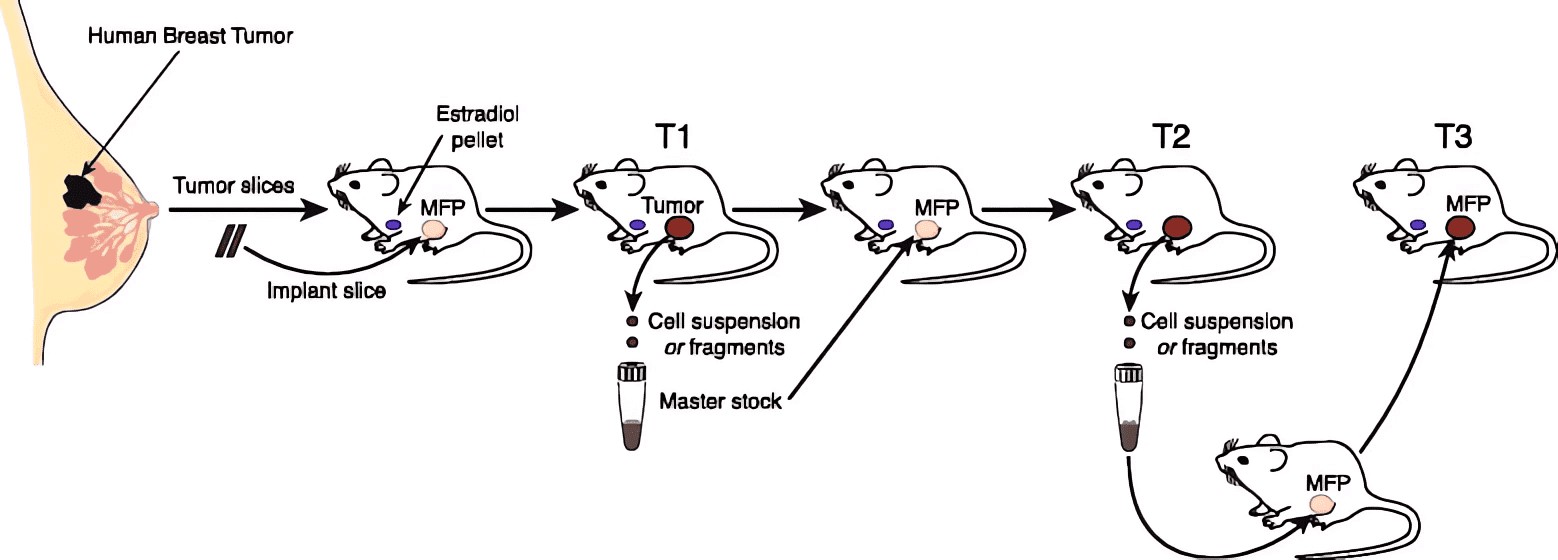 Figure 1 Process of breast cancer PDX model construction.1,3
Figure 1 Process of breast cancer PDX model construction.1,3
Disease Models
-
Diet-induced Obesity
The model can be used to evaluate the efficacy of ASO in vivo and the improvement of the drug on weight, fat content, serum cholesterol, and other indicators, so as to evaluate the therapeutic effect of the drug. Male mice were generally selected for modeling from the 6th week and fed 35% high-fat diet for 8-12 weeks. -
Myotonic Dystrophy (MD)
MD is a genetic disorder in which the muscles gradually atrophy and stiffen. Dmpk knockout mice were constructed, and the mice exhibited muscle weakness and heart disease, which shared some pathological features with MD. -
Psoriasis
Psoriasis is a common chronic inflammatory skin disease. The psoriatic mouse model can be generated by transplanting psoriatic skin lesions into immunodeficient mice, inducing the condition with drugs (e.g., imiquimod), and applying a restricted essential fatty acid diet, among others. -
Duchenne Muscular Dystrophy (DMD)
The most widely used animal model is the myodystrophin-deficient mdx mouse, which has a mutation in the anti-myodystrophin gene, resulting in reduced expression of fully functional myodystrophin. -
Huntington's Disease (HD)
R6/2 and YAC128 mice, as common animal models for the study of Huntington's disease, showed significant neuropathological changes in the caudate putrum and other brain regions, including hyperkinesia, gait abnormalities, movement disorders, neuron cell loss, and neurodegeneration. -
Spinal Muscular Atrophy (SMA)
In the SMA mouse model, the mouse Smn1 gene is replaced in situ by the human SMN2 gene. The loss of the SMN protein resulted in developmental deficits in SMA mice, characterized by muscle atrophy, unsteady standing, severed tail, and swollen limbs.
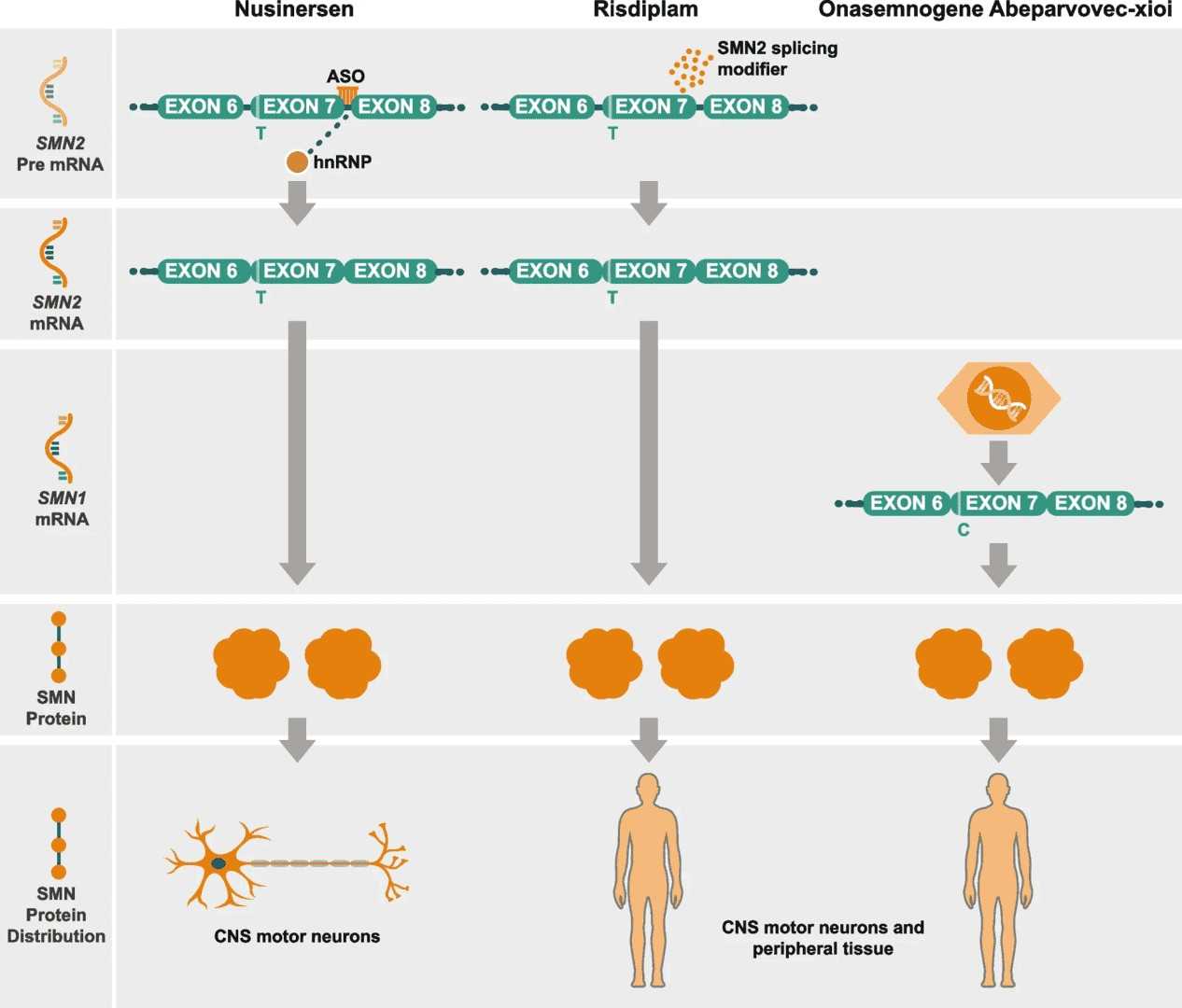 Figure 2 The pathogenesis of SMA.2,3
Figure 2 The pathogenesis of SMA.2,3
Our Services In Vivo Research Tests
After the animal model is established, a variety of parameters of indicators can be assessed. Creative Biolabs offers professional and custom analysis services, including custom-developed assays, data analysis, and detailed reports. The table below shows the commonly used detection indicators for in vivo studies.
Physicochemical Properties and Metabolism Detection of ASO
- Assess the effect of modification on ASO stability, determine an optimal modification scheme
- ASO metabolites detection
Delivery System Optimization and Bioavailability Assessment
- Detect the efficiency of delivery systems and provide optimization services
- Detect the effect of bioavailability affected by different administration route
Pharmacodynamics and Pharmacokinetics Detection
Pharmacodynamic properties of ASOs were evaluated by measuring their expression levels, gene regulation effects, and biological function changes in target tissues
Safety and Toxicity Assessment
- Acute and long-term toxicity detection
- Immunogenicity study detection
- Specific toxicity study detection
Species Differences and Preclinical Studies
Based on the results of preclinical studies, provide a scientific basis for clinical trials, and formulate a reasonable administration plan and dosage range.
Our Collaborative Process
We view every project as a scientific partnership. Our workflow is designed to ensure transparency, scientific rigor, and alignment with your specific drug development milestones.
-
Phase I: Consultation & Design
Our PhD-level scientists review your target biology and ASO chemistry. We select the optimal animal species and design the study protocol, including sample size calculation based on statistical power analysis.
-
Phase II: Model Generation & Validation
If a specific transgenic or humanized model is required to ensure sequence homology, we leverage our internal gene-editing capabilities to generate the appropriate strain.
-
Phase III: Execution & Dosing
Our veterinary staff performs precision administration via various routes (IV, SC, Intravitreal, or Intrathecal for CNS targets).
-
Phase IV: Analysis & Reporting
We perform comprehensive bioanalysis, including hybridization ELISAs, qPCR, and LC-MS/MS, delivering a final report ready for regulatory submission.
Customer Reviews
"The challenge with our ASO candidate was its delivery to the dorsal root ganglia. Creative Biolabs suggested a specific intrathecal dosing regimen in rats that perfectly mimicked the clinical route. Their PK data was instrumental in our pre-IND meeting with the FDA."
— Dr. Elena R., VP of Preclinical Development
"We needed to differentiate our GalNAc-ASO from a competitor based on duration of action. Creative Biolabs designed a long-term pharmacodynamic study that demonstrated our compound's superior potency over 8 weeks. The data quality was exceptional."
— Dr. Satoshi K., Senior Scientist
Frequently Asked Questions
Q: Can you perform studies for exon-skipping oligonucleotides?
A: Yes. We utilize specific assays such as RT-PCR with primers flanking the target exon to quantify the ratio of full-length vs. skipped transcripts in muscle or CNS tissues.
Q: How do you handle species specificity issues?
A: If your ASO does not cross-react with the rodent ortholog, we recommend using a surrogate ASO (designed for the mouse sequence) or utilizing our humanized mouse models where the human gene replaces the mouse gene.
Q: What is the typical timeline for a PK/PD study?
A: A standard rodent study (acclimatization, dosing, necropsy, and bioanalysis) typically takes 4–6 weeks, depending on the duration of the observation phase.
Q: Can you support studies with non-human primates (NHPs)?
A: Yes. We have extensive experience with NHP studies for advanced PK/PD and toxicology assessments, particularly for systemically administered ASOs targeting hepatic genes.
Q: How do you handle the bioanalysis of modified ASOs in tissues?
A: We employ state-of-the-art extraction techniques followed by sensitive analytical platforms (LC-MS/MS or hybridizing immunoassays). We develop and validate ASO-specific methods for each program to ensure accuracy.
Q: What are your capabilities for CNS-targeting ASOs?
A: We offer intracerebroventricular (ICV) and intrathecal (IT) dosing in rodents and larger species, with specialized necropsy and micro-dissection protocols for CNS tissue collection and analysis.
Connect with Us Anytime!
The path to a successful antisense therapeutic is paved with rigorous in vivo data. At Creative Biolabs, we combine deep scientific expertise in RNA biology with state-of-the-art animal research facilities to provide a clear view of your candidate's potential. Whether you are targeting a rare genetic disorder or a common metabolic disease, our team is dedicated to providing the actionable insights you need.
Reference
- Whittle, James R., et al. "Patient-derived xenograft models of breast cancer and their predictive power." Breast cancer research 17 (2015): 1-13. https://doi.org/10.1186/s13058-015-0523-1
- Day, John W., et al. "Advances and limitations for the treatment of spinal muscular atrophy." BMC pediatrics 22.1 (2022): 632. 10.1186/s12887-022-03671-x
- Distributed under Open Access license CC BY 4.0, without modification.
