Antisense Oligonucleotide (ASO) Off-Target Detection and Analysis Service
In the rapidly evolving landscape of genetic medicine, Antisense Oligonucleotides (ASOs) have emerged as a dominant modality for modulating gene expression. From the treatment of Spinal Muscular Atrophy (SMA) to rare metabolic disorders, ASOs offer a programmable approach to targeting the "undruggable" genome. At Creative Biolabs, we understand that the success of a therapeutic candidate is defined not just by what it targets, but by what it avoids. We employ an integrative, multi-omics approach combining state-of-the-art in silico prediction, transcriptome-wide experimental screening, and rigorous validation to deliver a comprehensive off-target profile.
ASO Gene Therapy
ASOs are short, synthetic, single-stranded oligodeoxynucleotides that can alter RNA processing and reduce target protein expression through multiple mechanisms, primarily RNase H-mediated cleavage or steric blockage of ribosomal machinery.
Ideally, an ASO binds exclusively to its complementary target pre-mRNA or mRNA via Watson-Crick base pairing. However, the human transcriptome contains vast repetitive sequences and partial homologies. This leads to two primary categories of off-target effects that must be scrutinized:
- Hybridization-Dependent Off-Targets: The ASO binds to unintended RNA transcripts with partial sequence complementarity, leading to the downregulation of essential non-target genes.
- Hybridization-Independent (Aptameric) Effects: The ASO binds to proteins or triggers immune responses (e.g., TLR activation), leading to toxicity independent of base pairing.
Ignoring these interactions can result in hepatotoxicity, nephrotoxicity, or thrombocytopenia, halting development in late-stage trials.
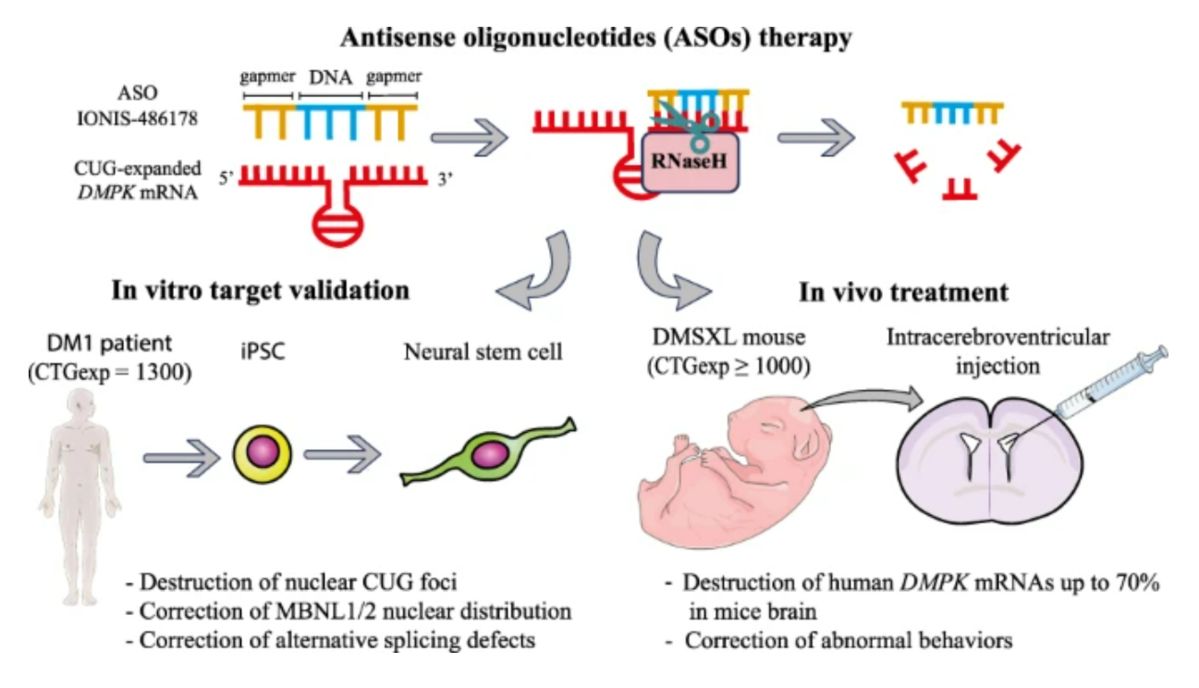 Figure 1. Antisense oligonucleotides (ASOs) therapy.1
Figure 1. Antisense oligonucleotides (ASOs) therapy.1
ASO Off-Target Effect
ASO drugs target pre-mRNA or mature mRNA and induce degradation to achieve therapeutic effects. However, due to sequence similarities between target mRNA and non-targeted mRNA, mismatch, insertion, and deletion can occur, leading to off-target effects, triggering unexpected genetic mutations or functional changes that affect study results or therapeutic efficacy, and may raise safety concerns.
Therefore, in the development process, it is important to understand the off-target effects of ASO drugs as early as possible. Through professional off-target effect detection and analysis services, drug developers can identify potential safety risks early in drug discovery and provide safer and more effective treatment options for patients.
Application Scenarios: Why is Off-Target Analysis Critical?
Our detection services are integral at multiple stages of the drug development lifecycle:
- Lead Optimization: Differentiating between multiple potent ASO candidates by selecting the one with the cleanest safety profile.
- Preclinical Toxicology: mechanistic investigation of observed toxicities in animal models to determine if they are on-target (exaggerated pharmacology) or off-target.
- Regulatory Submission: Providing comprehensive "safety packages" demonstrating that exhaustive measures were taken to map the interactome of the therapeutic candidate.
- Mechanism of Action (MoA) Validation: Confirming that phenotypic improvements are due to target knockdown and not ancillary pathways.
Commonly Used Off-target Effect Detection Method
Whole Genome Sequencing (WGS) Method
WGS is one of the simplest and most effective ways to detect off-target effects. By sequencing the entire genome, a wide range of mutations resulting from gene editing were comprehensively detected, including single nucleotide mutations (SNV), insertion-deletion variants (Indels), chromosome rearrangements, copy number variants (CNV), and structural variants (SV). This method is highly sensitive and comprehensive and can detect very off-target events of low frequency, but the cost is relatively high, the data analysis process is complex and requires the support of a professional bioinformatics team.
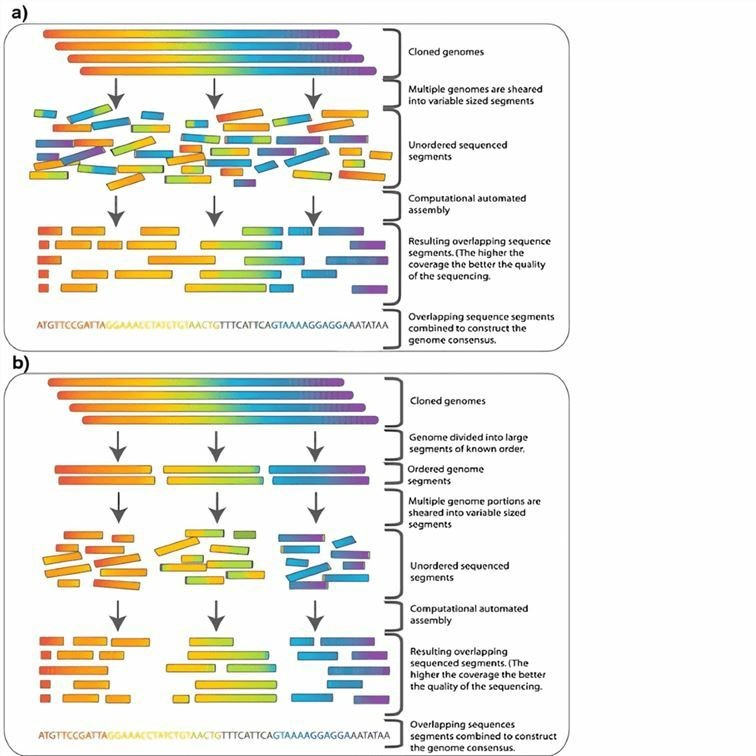 Figure 2. Whole genome sequencing diagram.Distributed under CC BY-SA 2.5, from Wiki, without modification.
Figure 2. Whole genome sequencing diagram.Distributed under CC BY-SA 2.5, from Wiki, without modification.
Target Capture and Sequencing Method
Target acquisition sequencing is a method for high-throughput sequencing of specific regions, and the acquisition probe is designed according to the predicted off-target sites for targeted sequencing. Compared with whole genome sequencing, this method has a lower cost and can detect specific off-target sites, improving the pertinence and accuracy of detection. However, coverage is limited and it may not be possible to detect all potential off-target sites.
Digenome-seq Method
Digenome-seq uses in vitro cutting technology to simulate the gene editing process, and sequencing the genomic DNA after cutting, comparing the genomic sequences before and after cutting, can identify off-target effects. The operation is simple and the cost is relatively low, but it may be affected by the efficiency and specificity of in vitro cutting.
PCR-based Detection Method
Specific endonucleases are used to cut PCR products, and then off-target sites are identified by analyzing the sequence of the cut products. The operation is simple and low-cost, but it may be affected by the amplification efficiency and specificity of PCR, and only off-target effects can be detected at specific locations.
Our Services
To thoroughly decipher and quantify the off-target risk of candidate therapeutics, we offer a comprehensive, tiered suite of ASO Off-Target Detection and Analysis solutions. Our services integrate cutting-edge bioinformatic prediction, transcriptome-wide experimental screening, and multi-level biochemical validation, forming a complete cycle. Instead, through multi-dimensional, orthogonal data that cross-validates each other, we deliver an off-target profile of high confidence that exceeds industry standards. This process transforms potential safety and mechanistic uncertainties into clear, actionable intelligence for your R&D decision-making.
The Highlight of Our Service
Creative Biolabs offers a comprehensive range of ASO drug off-target testing services, including:
- Target screening and Validation: Use advanced bioinformatics tools to screen and identify potential sequences on the human genome that overlap with ASO or target genes to ensure accuracy.
- Off-target Effect Evaluation: Comprehensive evaluation of off-target effects of drug candidates through functional screening methods. Creative Biolabs focuses on the silencing effect of drugs on non-target genes, as well as possible biological effects.
- Reporting and Consulting: Detailed presentation of test results and data analysis. The team of experts will provide professional advice and recommendations to help customers develop subsequent research and development strategies.
- Comprehensive Coverage: Creative Biolabs' services cover the whole process from target screening to off-target effect assessment, ensuring full and accurate identification of potential risks.
- Professional Team: Creative Biolabs has a team of experienced experts with a deep background in biology, able to provide customers with professional technical support and solutions.
- Reliability of Data: All targets are tested using functional methods, which can reduce false negatives and avoid safety risks in the later stage to a greater extent.
Result Delivery
| Deliverable Component | Detailed Description |
|---|---|
| Comprehensive Study Report | A fully annotated document containing the executive summary, detailed materials & methods (suitable for regulatory filing), experimental protocols, Quality Control (QC) metrics, and statistical methodology. |
| Raw & Processed Data | Full access to the underlying data generated during the study. This includes raw sequencing reads, aligned binary files, and normalized gene expression matrices. |
| Data Visualization Suite | Publication-ready figures illustrating the results. Includes Volcano Plots (significance vs. fold change), Hierarchical Clustering Heatmaps, and Gene Ontology (GO) enrichment graphs. |
| Risk Assessment Analysis | A tiered classification of identified off-targets. We categorize hits based on sequence homology, thermal stability, and biological relevance (tissue-specific expression), providing a clear "Go/No-Go" safety recommendation. |
| Validation Data (Optional) | If qPCR validation was requested, we provide the raw Ct values, amplification curves, and relative quantification analysis (ΔΔCt method) for top off-target hits. |
Our Collaborative Process
We view our clients as scientific partners. Our workflow is designed to be transparent, rigorous, and adaptive to your specific therapeutic modality.
-
Consultation & Design
We begin with a deep dive into your ASO chemistry (PS-backbone, sugar modifications), sequence, target gene, and intended therapeutic context.
-
Customized Workflow Development
Based on the consultation, we propose a tailored strategy, selecting from our suite of bioinformatic and experimental tools.
-
Execution & Iterative Analysis
We conduct the agreed-upon experiments while maintaining open communication on interim findings, allowing for adaptive investigation of initial results.
-
Reporting & Consultation
We deliver a final, interpretative report and host a detailed results review session to discuss implications for your program.
Frequently Asked Questions
Q: How early should I incorporate off-target analysis into my ASO program?
A: Ideally at the lead selection stage. Screening 3-5 candidate sequences can identify the one with the cleanest profile before committing significant resources to preclinical development.
Q: Can you analyze all ASO chemical modalities?
A: Yes. Our protocols are optimized for various chemistries including phosphorothioate (PS)-backbone gapmers, fully modified steric-blocking ASOs (e.g., 2'-MOE, PMO), and siRNA duplexes.
Q: Do you support different ASO chemistries?
A: Yes, we have extensive experience with Phosphorothioate (PS), 2'-O-Methoxyethyl (2'-MOE), Locked Nucleic Acid (LNA), Morpholino (PMO), and stereopure oligonucleotides.
Q: Can you perform analysis in non-human species?
A: Absolutely. We frequently perform off-target analysis in mouse, rat, and cynomolgus monkey genomes to support preclinical safety studies.
Q: How sensitive is your RNA-seq detection?
A: Our optimized library prep and deep sequencing (minimum 40M paired-end reads/sample) can reliably detect splicing changes and expression differences as low as 1.5-fold.
Connect with Us Anytime!
Through its professional off-target effect detection services, Creative Biolabs helps drug developers detect off-target effects of ASO drugs as early as possible and reduce potential risks, ensuring their safety and efficacy. If you are developing an ASO drug and would like to learn more about the detection of off-target effects, please contact us.
Reference
- Ait Benichou S, Jauvin D, De Serres-Bérard T, et al. Antisense oligonucleotides as a potential treatment for brain deficits observed in myotonic dystrophy type 1. Gene Therapy, 2022, 29(12): 698-709. https://doi.org/10.1038/s41434-022-00316-7 (Distributed under Open Access license CC BY 4.0, without modification.)
