AAV Vector Design Service for Alzheimer's Disease (AD)
Introduction
Developing effective, long-term Alzheimer's disease treatments faces hurdles like targeted gene delivery to the central nervous system and challenges in preclinical/clinical research progress. Creative Biolabs' custom rAAV design service addresses these via optimized, high-titer AAV vectors—engineered with advanced recombinant DNA tech for precise CNS delivery and robust expression. It provides a comprehensive solution to accelerate AD therapeutic development, speed timelines, and support regulatory submissions.
Discover How We Can Help - Request a Consultation
Alzheimer disease
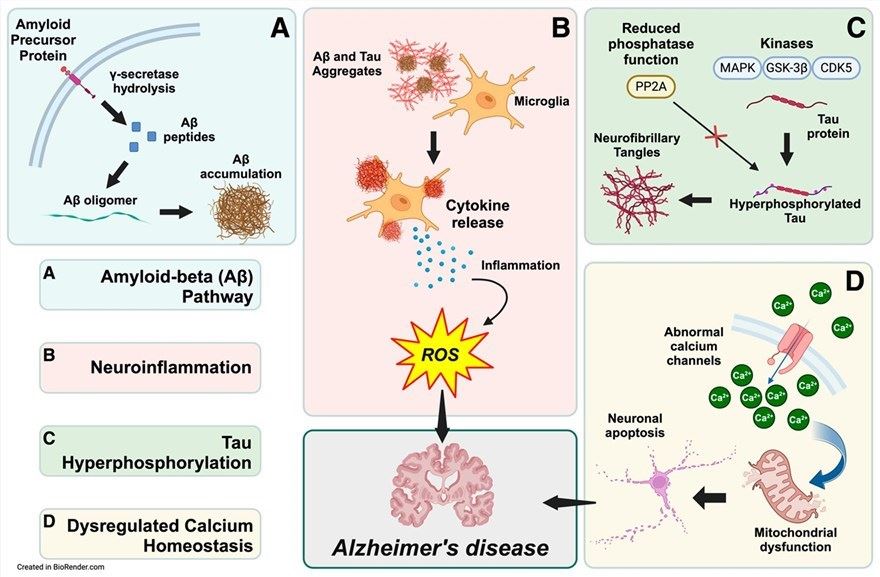 Fig.1 Biochemical pathways of the pathogenesis of Alzheimer's disease.1
Fig.1 Biochemical pathways of the pathogenesis of Alzheimer's disease.1
Alzheimer's disease (AD), the most common cause of dementia, is marked by progressive neurodegeneration, with its pathology centered on the accumulation of amyloid-beta (β) plaques and neurofibrillary tangles (composed of hyperphosphorylated tau protein)—these accumulations drive neuronal dysfunction and death. The "amyloid cascade hypothesis" posits that imbalanced amyloid-beta production and clearance is the core pathogenic event. Current treatments like cholinesterase inhibitors and NMDA receptor antagonists only provide temporary symptomatic relief without halting progression, while the emergence of gene therapy offers a transformative approach: it targets AD's underlying genetic and protein-level causes, shifting from symptomatic management to disease modification.
AD's primary therapeutic targets are amyloid-beta (Aβ) and tau proteins—gene therapy targets these at the source to slow/stop neurodegeneration, with strategies including delivering genes for Aβ-clearing antibodies, Aβ-breaking enzymes, neurotrophic factors (e.g., BDNF), or tau phosphorylation reducers.
AAV-based treatments lead this approach: AAV vectors (some crossing the blood-brain barrier) enable long-term CNS gene expression. Engineered to carry therapeutic genes, they address AD's root causes; this single-shot method may offer durable effects, avoiding frequent administration for true disease modification.
Workflow
- Required Starting Materials: To initiate a project, clients typically provide a target gene sequence (e.g., for antibody expression, a neurotrophic factor, or an enzyme), a clear statement of project goals (e.g., amyloid clearance or tau pathology reduction), and any preliminary in vitro or in vivo data available.
-
Step 1: Consultation & Strategy
Start with a detailed consultation to grasp project needs/objectives; co-design a customized gene therapy strategy (vector design, serotype selection, promoter optimization) for maximum efficacy and tissue specificity. -
Step 2: Vector Design & Construction
Use advanced recombinant DNA tech to design/construct rAAV vectors with target genes, via meticulous cloning and sequence verification. -
Step 3: High-Titer Virus Packaging
Package vectors into optimized AAV serotypes for high-titer/purity particles; use proprietary suspension culture and scalable purification for consistent batches (preclinical/clinical use). -
Step 4: Quality Control & Validation
Conduct rigorous QC (qPCR for titer, SDS-PAGE for purity/protein integrity, in vitro assays for transduction efficiency/gene expression). -
Step 5: Final Deliverables & Data Handover
Deliver purified rAAV vectors and full data package (QC reports, project summary, methodology docs).
- Estimated Timeframe
Typically, 8-16 weeks, depending on gene complexity, project scope, construct difficulty, serotype choice, and production scale.
What We Can Offer
At Creative Biolabs, we are dedicated to providing customized gene therapy solutions that empower your research. Our integrated platform offers a comprehensive suite of services, designed to meet the unique needs of your Alzheimer's disease project.
One-Stop Gene Therapy Services
Offer end-to-end support from lab-scale proof-of-concept to large-scale GMP production, ensuring consistency and speeding up project timelines.
Customized AAV Design & Optimization
Experts optimize gene codon usage for better host cell expression, guarantee construct stability, and adjust production modes to maximize therapeutic yield.
High-Purity and High-Titer Production
Use a scalable platform with over 100,000L total bioreactor capacity to supply reliable, high-titer, high-purity vectors.
Rigorous Quality System
Operate with QbD, PAT, strict aseptic verification, and GMP compliance to ensure top product quality and safety.
Comprehensive Quality Control
Employ high-standard tools for product quality evaluation, plus full strain origin documentation for traceability and result confidence.
Customer Reviews

FAQs
How do your AAV vectors specifically target neurons in the brain for AD therapy?
We use proprietary AAV serotypes that have been optimized for CNS tropism. This allows our vectors to efficiently transduce neurons and other relevant cells after direct injection, ensuring that the therapeutic payload reaches its intended cellular target for maximum effect.
Can your service handle projects targeting both amyloid and tau pathology?
Yes, our platform is highly versatile. We can design and produce AAV vectors for both anti-amyloid strategies (e.g., expressing antibodies or enzymes) and anti-tau strategies (e.g., delivering genes for kinase inhibitors). This allows us to support a wide range of therapeutic strategies.
What makes your AAV production process more efficient and cost-effective than others?
We have developed a proprietary suspension cell culture and purification platform that dramatically increases the yield and purity of our AAV vectors. This high-throughput process reduces costs and ensures consistency between batches, which is essential for both research and clinical applications.
At Creative Biolabs, we are committed to providing the highest quality rAAV design and production services to accelerate your research and bring innovative AD therapies to patients faster. Our expertise in neurodegenerative diseases and advanced vector technology makes us the ideal partner for your next project.
Contact Our Team for More Information and to Discuss Your Project
References
- Kazemeini, Sarah, et al. "From plaques to pathways in Alzheimer's disease: the mitochondrial-neurovascular-metabolic hypothesis." International Journal of Molecular Sciences 25.21 (2024): 11720. https://doi.org/10.3390/ijms252111720. Distributed under Open Access license CC BY 4.0, without modification.
