Liposomes have great potential for precise drug delivery and therapeutics that can be used to deliver drugs, genes, and vaccines. Therefore, a comprehensive assessment of toxicity for liposomes can ensure their safety in clinical applications. Creative Biolabs is committed to the development of high-quality liposomes, and we have always emphasized that three important features in drug-loaded liposome development are safety, efficacy, and controllable quality. Over years of dedicated effort, we have built a comprehensive liposome platform. Whether for new liposome development or improvement of existing products, we provide reliable solutions and data analysis for safety assessment.
Preclinical toxicology studies of liposomes can predict their potential risks or side effects. These require considering not only the toxicity from encapsulated drugs but also the possible toxicity of carriers. In general, the toxicity of the whole formulation is studied without characterizing the liposomes themselves, failing to distinguish between drug and carrier. Therefore, the toxicity of empty liposomes (nondrug-loaded) should be particularly emphasized. Their potential harm is manifested by a persistent accumulation at the delivery site, ultimately leading to chronic inflammation. Furthermore, the toxicological profile of bulk materials cannot be relied upon to directly define the toxicity of a liposomal delivery system, but rather a combination of factors needs to be considered. Therefore, the safety evaluation of liposomes needs to be performed on a case-by-case basis.
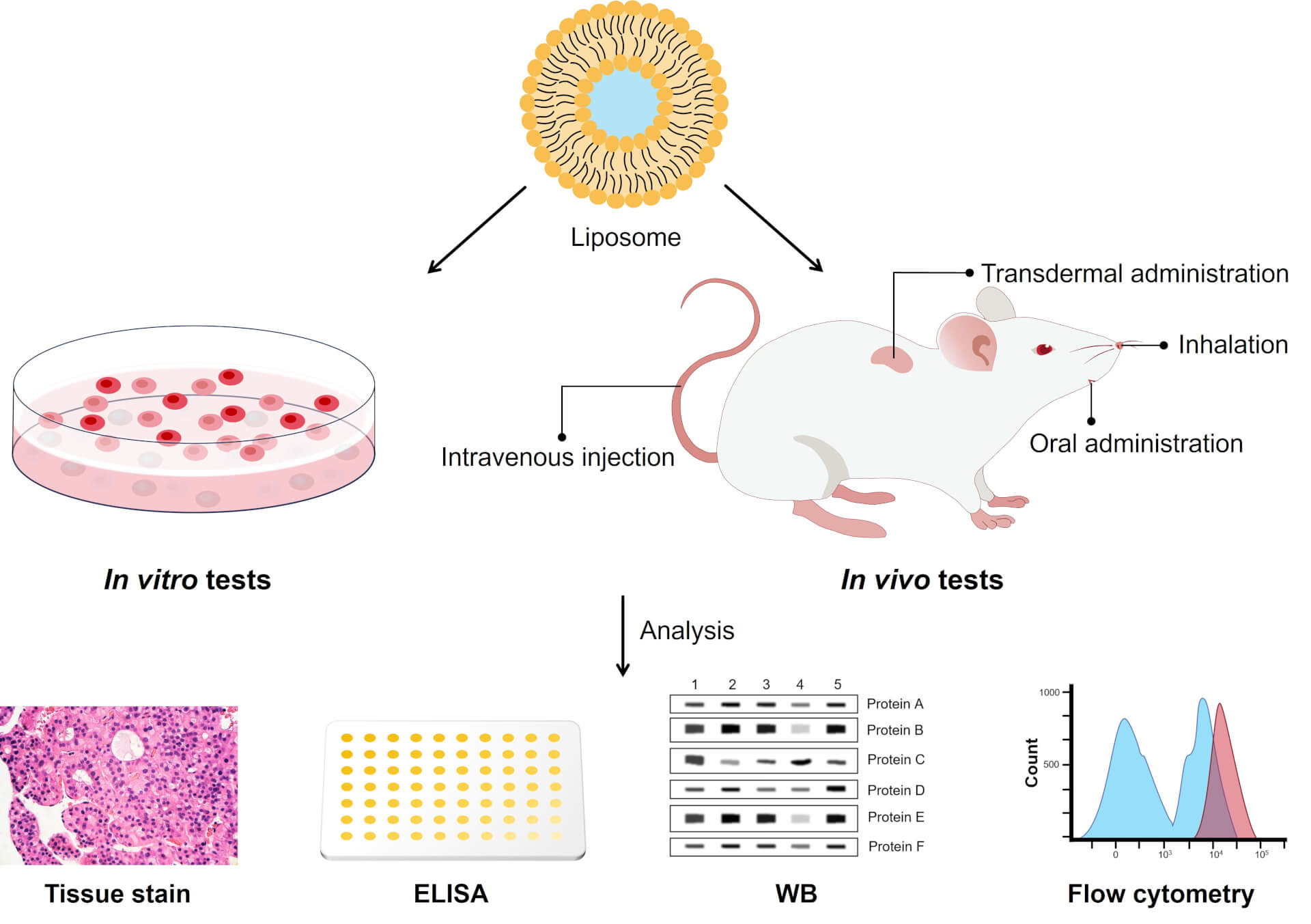 Fig 1. Safety Evaluation in vitro and in vivo.
Fig 1. Safety Evaluation in vitro and in vivo.
General toxicity assessment determines the potential toxic effects of liposomes over a range of doses and different durations of administration. This can be used to evaluate the systemic and local toxicity of liposomes. We offer the following methods for assessing general toxicity.
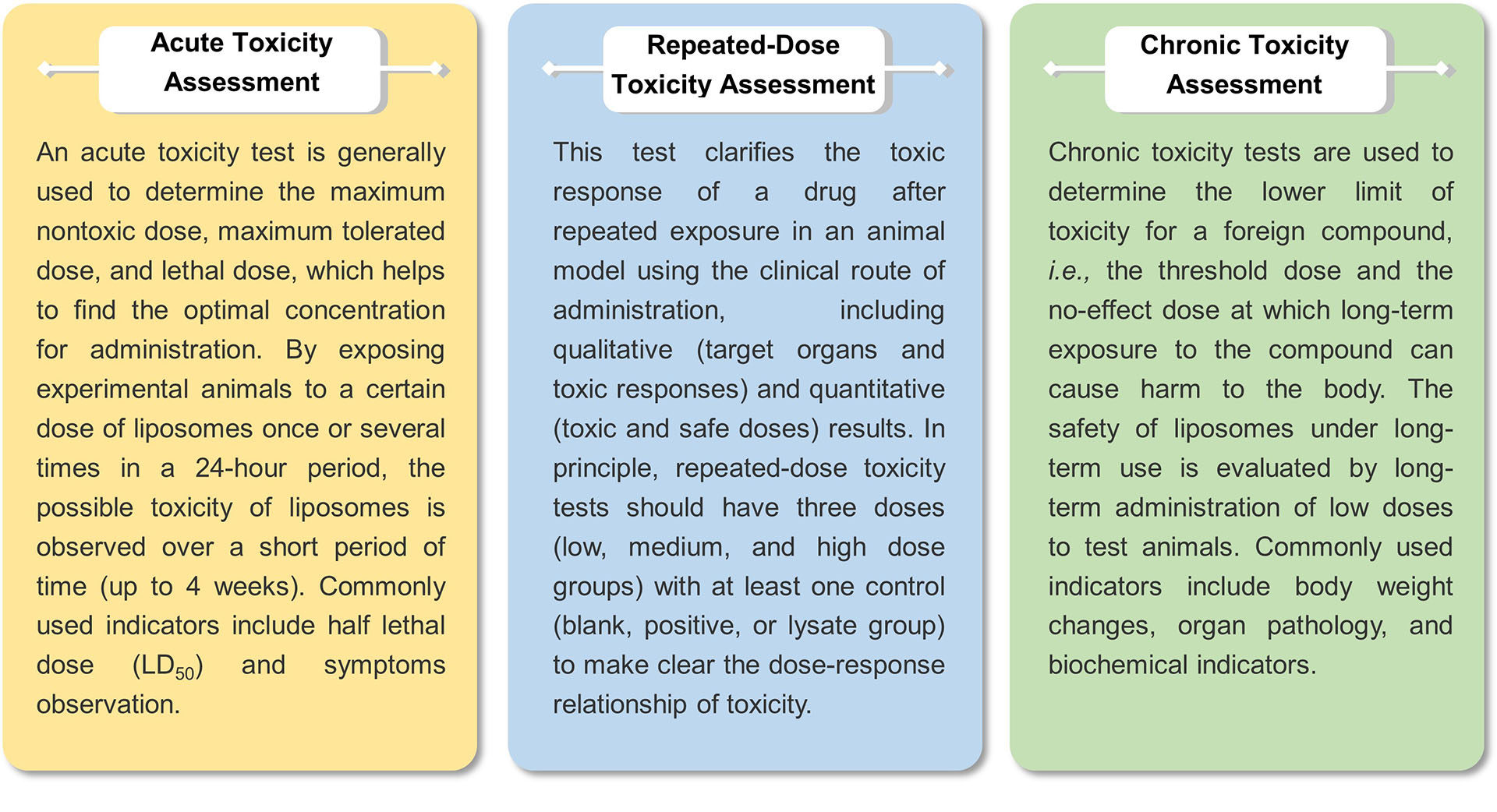
The ability of liposomes to cause cell damage or death can be measured by in vitro cytotoxicity studies. We offer toxicity assays for cytotoxicity biomarkers (e.g., lactate dehydrogenase and glucose 6-phosphate dehydrogenase), cell viability, autophagy, apoptosis, and lipotoxicity. Commonly used cell lines include human hepatocytes (HepG2) and lung epithelial cells (A549).
Furthermore, we also provide allergenicity assessments and immunogenicity assessments. If you are interested in our safety studies or have any questions, please feel free to contact us. Our experts in liposomes will be dedicated to providing you with professional consultation and support.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseServices
Online Inquiry