Liposomes for Immune Activation
Background Liposome-based Vaccine Services Platforms Advantages Related Services FAQs Resources
Liposomes are crucial lipid-based carriers with high biocompatibility and customizable designs, essential in advancing subunit and mRNA vaccine research. Known for enhancing immune activation, they play a key role in antigen delivery and adjuvant development. Creative Biolabs has established both drug delivery platform and vaccine platform, and combined the two to form a lipid-based drug delivery system in vaccines. By harnessing liposomes' potential, we support cutting-edge vaccine research
with unmatched precision and reliability.
Background of Liposome-based Immune Activation
Classical vaccines are generally whole killed or attenuated pathogens. In recent years, subunit vaccines are attracting extensive attention due to the advantages of better defined, easier to produce, and safer. These vaccines are produced based on well-characterized
antigens, such as recombinant proteins and peptides. However, their immunogenicity is not strong enough to induce the maturation of dendritic cells (DCs) due to their synthetic nature, and thus often leads to a weak immune response. Nanoparticulate
carriers such as liposomes can act as adjuvants for the enhancement of antigen delivery or activation of the innate immune response. The mechanisms by which liposomes enhance immune response include:
-
Antigen delivery, liposomes can help penetrate the tissues and access the lymphatics.
-
Depot effects, improved stability and sustained release of vaccine antigens.
-
Repetitive antigen display, facilitate B-cell receptor coaggregation, triggering, and activation.
-
Cross presentation, leakage of antigens into the cytosol may lead to the activation of major histocompatibility type I (MHC-I) pathways.
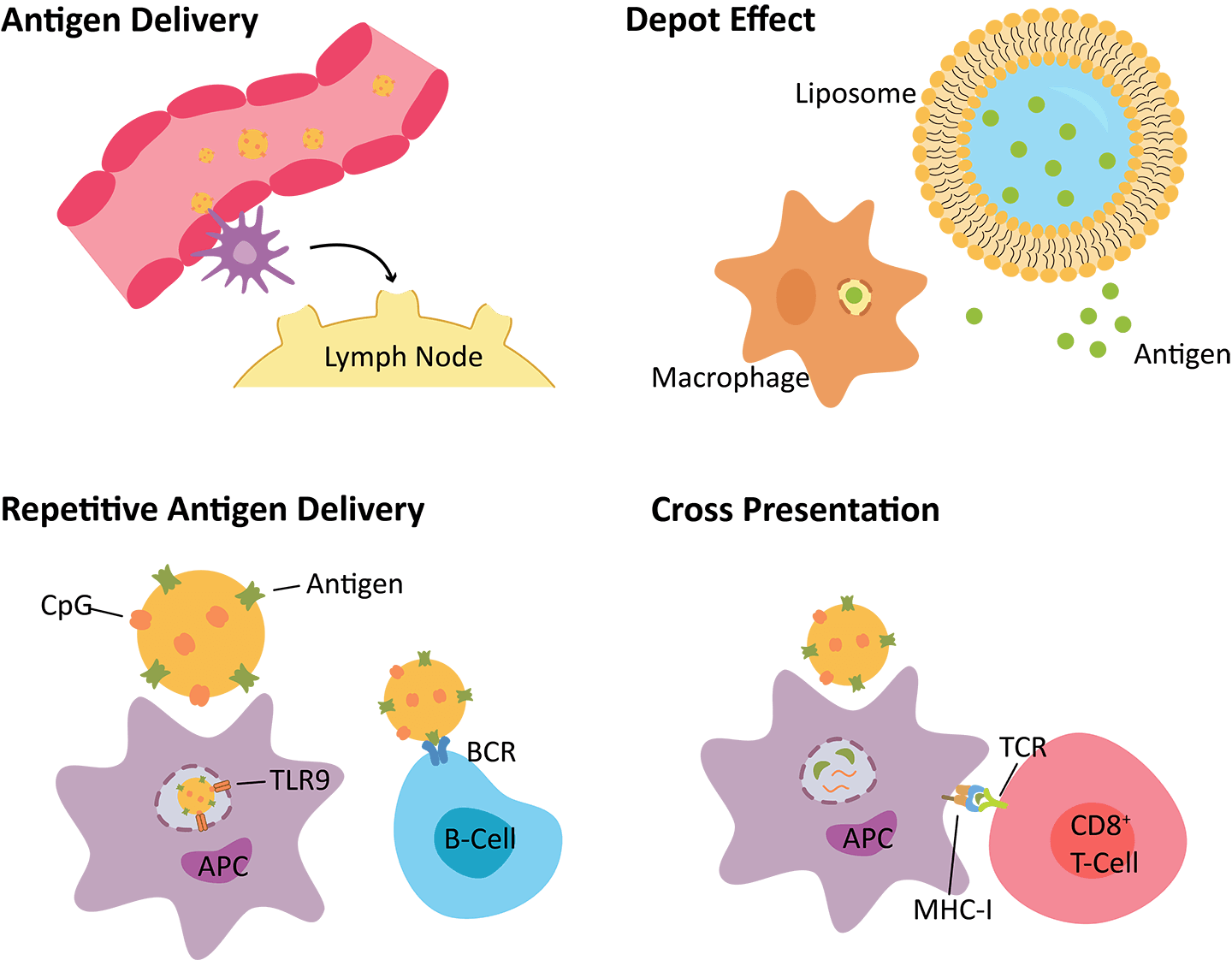 Fig.1 Mechanisms by which liposomes alter the induction
of immune responses.
Fig.1 Mechanisms by which liposomes alter the induction
of immune responses.
Liposome-based Subunit Vaccine VS Liposome-based mRNA Vaccine
|
Feature
|
Liposome-based Subunit Vaccine
|
Liposome-based mRNA Vaccine
|
|
Antigen Type
|
Specific antigenic components like proteins or peptides, lacking whole pathogens
|
mRNA constructs encoding specific antigens
|
|
Role of Liposomes
|
Enhances stability of antigenic molecules and boosts immunogenicity
|
Delivers mRNA safely into cells, ensuring high cellular uptake without integration risk
|
|
Immunostimulatory Molecules
|
Incorporates functional molecules, such as TLR (Toll-like receptor) and NLR (NOD-like receptor) agonists, to enhance immune response
|
Delivers non-replicating (NRM) or self-amplifying (SAM) mRNA constructs for different immune response pathways
|
|
Advantages
|
Provides improved antigen delivery and stabilization for more effective immune response in research applications
|
Stable, safe, and effective cellular uptake, promoting high translation efficiency and immune activation for vaccine research
|
|
Applications
|
Used extensively in developing subunit vaccines, improving immunogenicity without whole pathogen exposure
|
Supports mRNA vaccine studies, where rapid and efficient antigen expression is essential
|
|
Research Support by Creative Biolabs
|
Designs liposomes with integrated immune-stimulating molecules to enhance subunit vaccine efficacy
|
Offers encapsulated mRNA delivery solutions in liposomes for high-translational efficiency, supporting both non-replicating and self-amplifying mRNA constructs in vaccine research applications
|
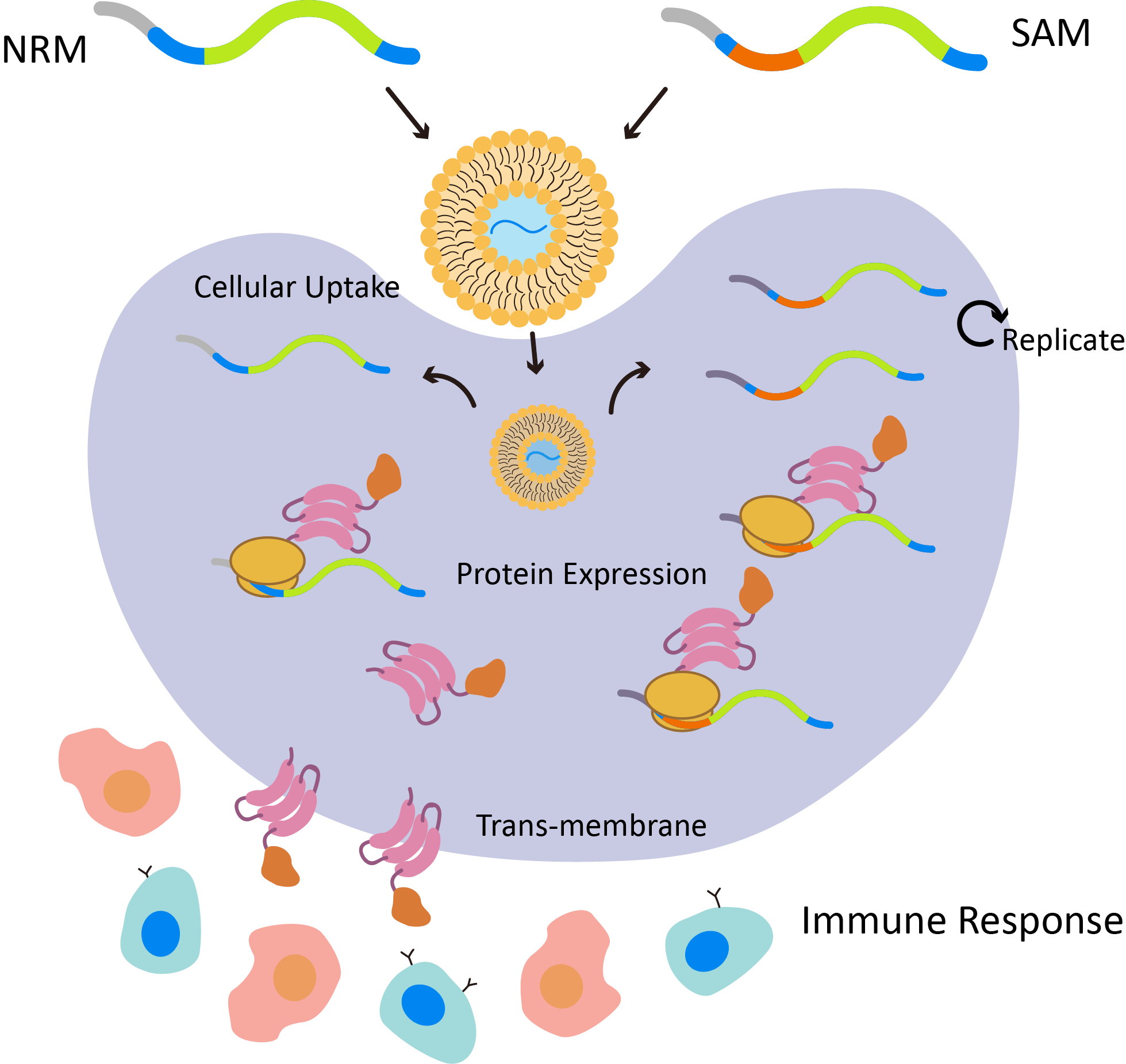 Fig.2 Mechanisms of liposomal mRNA vaccine.
Fig.2 Mechanisms of liposomal mRNA vaccine.
Liposome-Based Immune Activation Services
Creative Biolabs offers a comprehensive range of liposome-based immune activation services, tailored to the needs of modern research applications. Our service offerings are designed to provide end-to-end support for liposome-based vaccine development
and immune system research.
-
Utilizing advanced lipid formulation techniques, we create tailored liposome designs to accommodate various bioactive agents. Each liposome structure is optimized for specific applications, including size, surface charge, and encapsulation efficiency.
Preclinical Vaccine Development Platform
-
From antigen selection to adjuvant design, we support each stage of preclinical vaccine research. Our platform offers comprehensive solutions to ensure efficient antigen delivery and immune activation in in vitro and in vivo models.
-
We provide robust quality assessment tools, including formulation stability evaluation and safety testing, ensuring that each liposome formulation meets rigorous research standards.
Scientific Support
-
Our team of dedicated scientists provides technical expertise to support research objectives, from project design to troubleshooting, ensuring a seamless research process and optimal project outcomes.
Technical Platform Support
Creative Biolabs' technical platform offers end-to-end support for liposome research, ensuring each formulation meets precise research standards
Liposome Assembly and Characterization Platform
-
Equipped with advanced tools for nano-assembly, particle size control, and surface modification, enabling precise liposome designs tailored to various research needs.
-
Uses dynamic light scattering (DLS) and zeta potential analysis to monitor liposome stability in real-time.
Vaccine and Drug Testing Platform
-
Provides comprehensive in vitro and in vivo testing to evaluate vaccine efficacy and pharmacokinetics, essential for preclinical studies.
-
Includes immune response assessments and biodistribution analysis to support vaccine and drug research.
Quality Control Platform
-
A rigorous quality control process utilizing high-performance liquid chromatography (HPLC), transmission electron microscopy (TEM), and mass spectrometry to ensure the highest standards in consistency, stability, and research efficacy.
Advantages of Our Liposome-Based Immune Activation Services
Creative Biolabs is committed to delivering unmatched value in liposome-based research services by providing the following advantages:
-
One-Stop Service Experience: We cover every stage of the development process, from concept validation, design, and manufacturing to final testing, offering a seamless service experience.
-
High Quality Standards: Our quality control framework ensures that each liposome formulation meets stringent research standards for stability and consistency.
-
Customized Solutions: We provide flexible, customizable solutions to match specific research goals, accommodating unique research projects across various fields.
-
Experienced Team: Our scientific team has extensive experience in liposome development and immune activation, providing valuable insights and support throughout your project's lifecycle.
Related Services
To further support comprehensive research initiatives, Creative Biolabs offers a variety of related services, enhancing the scope of liposome-based applications:
FAQs
How do liposomes differ in subunit and mRNA vaccine applications?
Liposomes in subunit vaccines stabilize and protect antigens, while in mRNA vaccines, they enable high translational efficiency and safe cellular uptake, without risk of genetic integration.
What information is needed to start a liposome project?
Basic project details like target application, desired liposome characteristics, and specific requirements are needed to begin.
How does the testing phase work for immune activation applications?
In vitro and in vivo testing is performed to evaluate immune response and biodistribution, with data provided to clients for further analysis.
How is quality assurance handled throughout the service process?
Each stage, from design to final production, includes rigorous quality checks, ensuring consistency and stability in the liposome formulations.
Resources

For Research Use Only. Not For Clinical Use
 Fig.1 Mechanisms by which liposomes alter the induction
of immune responses.
Fig.1 Mechanisms by which liposomes alter the induction
of immune responses.
 Fig.2 Mechanisms of liposomal mRNA vaccine.
Fig.2 Mechanisms of liposomal mRNA vaccine.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical Use