Proper design, implementation, monitoring and evaluation of studies are essential for obtaining useful and accurate data on the stability of pharmaceutical formulations. The analytical platform of Creative Biolabs can complete the multi-dimensional stability test of liposome preparation, and we also have professional scientists to design the optimal stability evaluation method for you.
Liposomes have been widely used in drug delivery, drug targeting, controlled release and solubility enhancement. However, the main limitation of the widespread use of this universal drug delivery system is its instability. Therefore, the successful introduction of liposome formulations depends on a rigorous stability study.
The stability of liposomes is the major consideration in the production and administration of liposomes: from process to storage and delivery. Stable drug formulations maintain their physical integrity and do not adversely affect the chemical integrity of active ingredients during their life cycle. The stability study must include a description of the product characteristics and another part concerning the stability of the product during storage.
Integrated with other advanced platforms, Creative Biolabs provides the most comprehensive products and services including liposome stability analysis. Physical, chemical and microbiological parameters must be considered and evaluated when designing stability studies.
The particle size of all liposome preparations is not uniform, and the average particle size distribution of liposomes varies with storage time. Liposomes tend to fuse and grow into larger vesicles, which is a thermodynamically more favorable state. The fusion and rupture of liposomes during storage is also a key problem leading to drug leakage from vesicles. Therefore, particle morphology and size distribution are important parameters to evaluate physical stability.
The main component of liposome formulations is lipids, which are derived from natural and/or synthetic phospholipid sources containing unsaturated fatty acids that are known to undergo oxidation reactions. Therefore, it is necessary to develop a stability program to evaluate the chemical integrity of the drug over a period of time. There are many ways to monitor lipid degradation. For example, thin-layer chromatography (TLC) can be used to measure the purity and concentration of lipids.
Most therapeutic liposomes are non-intestinal products and must be sterilized to remove microbial contamination from the products. Therefore, it is very important to control the microbial stability of liposome formulations.
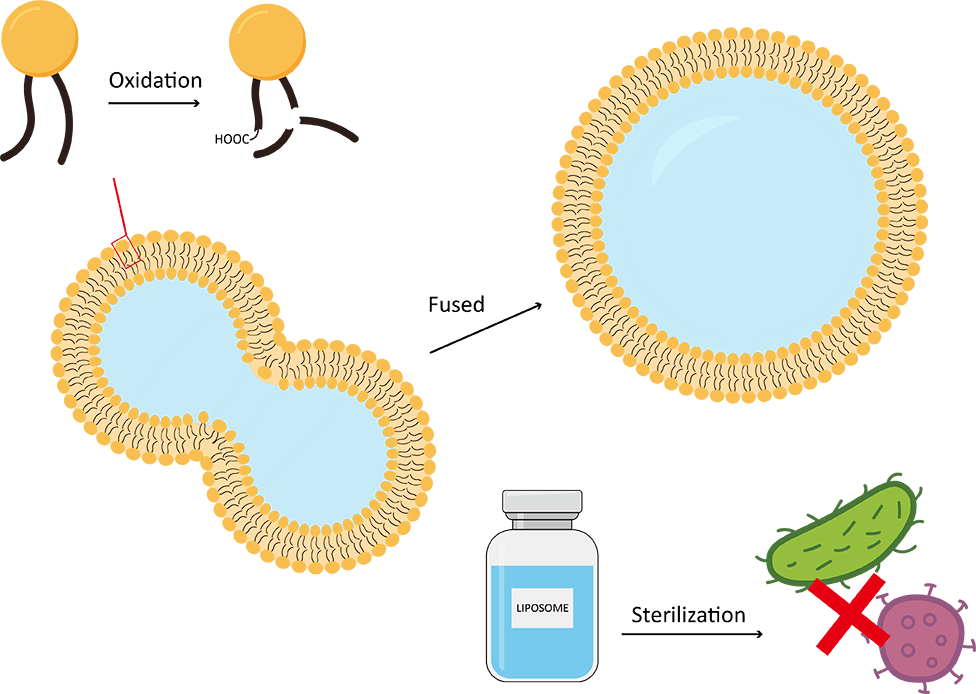 Fig.1 Liposome formulation stability monitoring. (Creative Biolabs)
Fig.1 Liposome formulation stability monitoring. (Creative Biolabs)
Our programs and service packages are available at competitive prices and fast turnaround times. For more details, please feel free to contact us or send us an inquiry directly.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseServices
Online Inquiry