Liposomes offer a promising approach for encapsulating one or more active ingredients within an aqueous core and/or membrane. In the development of liposomes, in vitro release (IVR) profiling is a fundamental point in the assessment of liposome delivery systems. The process of IVR testing involves exposing drug carriers to release media, such as saline or buffers, to simulate the drug release process in vivo. Liposomes can be customized to some extent based on the specific encapsulations and release conditions. The administration route (e.g., injection, infusion, or inhalation), the administration frequency (e.g., twice daily), and the expected IVR rate should be considered in the design of liposomes to maintain the efficacy and safety of the drug. Therefore, obtaining a reasonable IVR profile is important for a successful liposome delivery system.
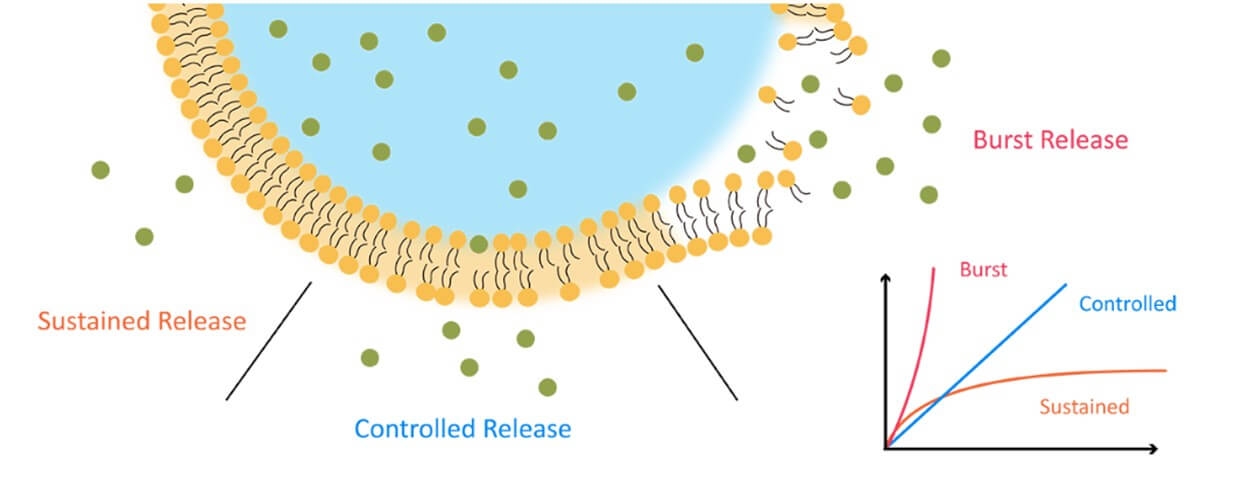 Fig.1 The mechanisms of drug release.
Fig.1 The mechanisms of drug release.
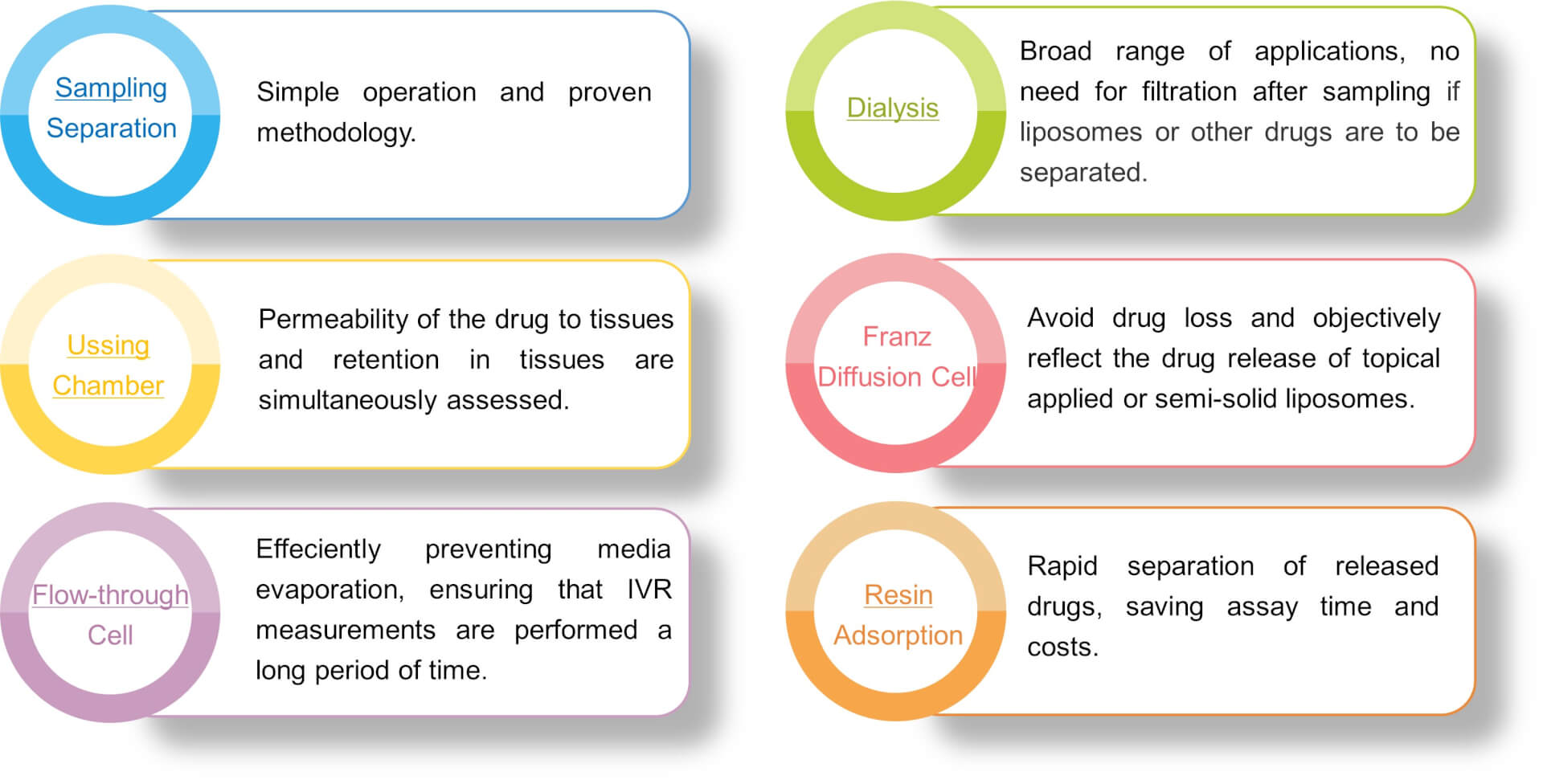
Creative Biolabs offers various separation techniques to evaluate the amount of drug released.
In addition, we offer several methods for quantifying released substances to calculate IVR curves, including spectrophotometry, enzyme assays, gel electrophoresis, high-performance liquid chromatography (HPLC), fluorescence spectroscopy, and field flow fractionation. Which method is chosen depends on the natural properties of the encapsulated drug.
The appropriate type of release media, temperature, and rotational speed are critical factors in establishing a method for liposome IVR. In addition, the dialysis method requires attention to the effects produced by the material, molecular weight cut-off, shape, size, and pretreatment. With many years of experience, our scientists at Creative Biolabs have established reproducible, operable, and standardized methods for IVR tests. We offer customized release kinetics services based on your specific formulation type, physicochemical properties, and administration route. For example, for the studies of oral liposomes, we typically use artificial gastric and intestinal fluids as the release media. If you are interested in our IVR services, please do not hesitate to contact us for more details.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseServices
Online Inquiry