Characterization of Lamellarity
31P nuclear magnetic resonance (31P NMR) is one of the most accurate methods to determine the lamellarity of the lipid layer. Small-angle X-ray scattering (SAXS) is used to measure the average lamellarity of multivesicular liposomes, the average thickness of interfacial layers, and the homogeneity of the electron density distribution in the layer. Furthermore, negative stain electron microscopy and cryo-microscopy are frequently combined with the aforementioned techniques to yield fast and efficient information about the average number of bilayers in multivesicular liposomes.
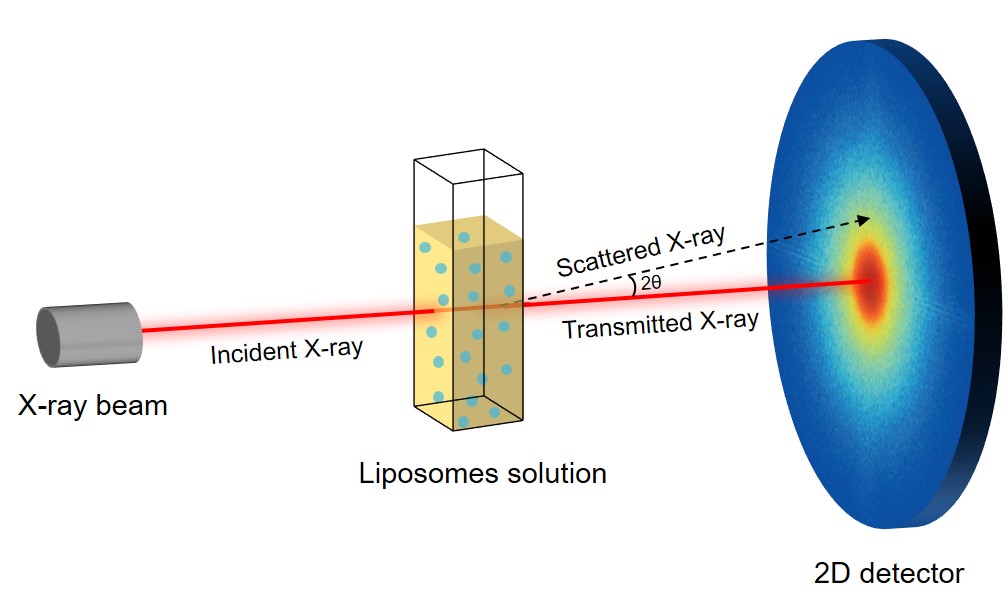 Fig.2 Principle of the SAXS method.
Fig.2 Principle of the SAXS method.
Characterization of Phase Behavior
Characterization by micropipettes, atomic force microscopy (AFM), spectroscopy of shape fluctuations, and optical dynamometry can offer relevant information on the phase behavior of liposomes. Also, the optical birefringence of the lipid layer can be measured by bipolarization interferometry to analyze the ordering and destructiveness associated with interactions or environmental effects. Differential scanning calorimetry (DSC) data can be used to experimentally determine the melting temperature (Tm). Pre- and main-phase transitions are evidently present in the heat capacity curve.
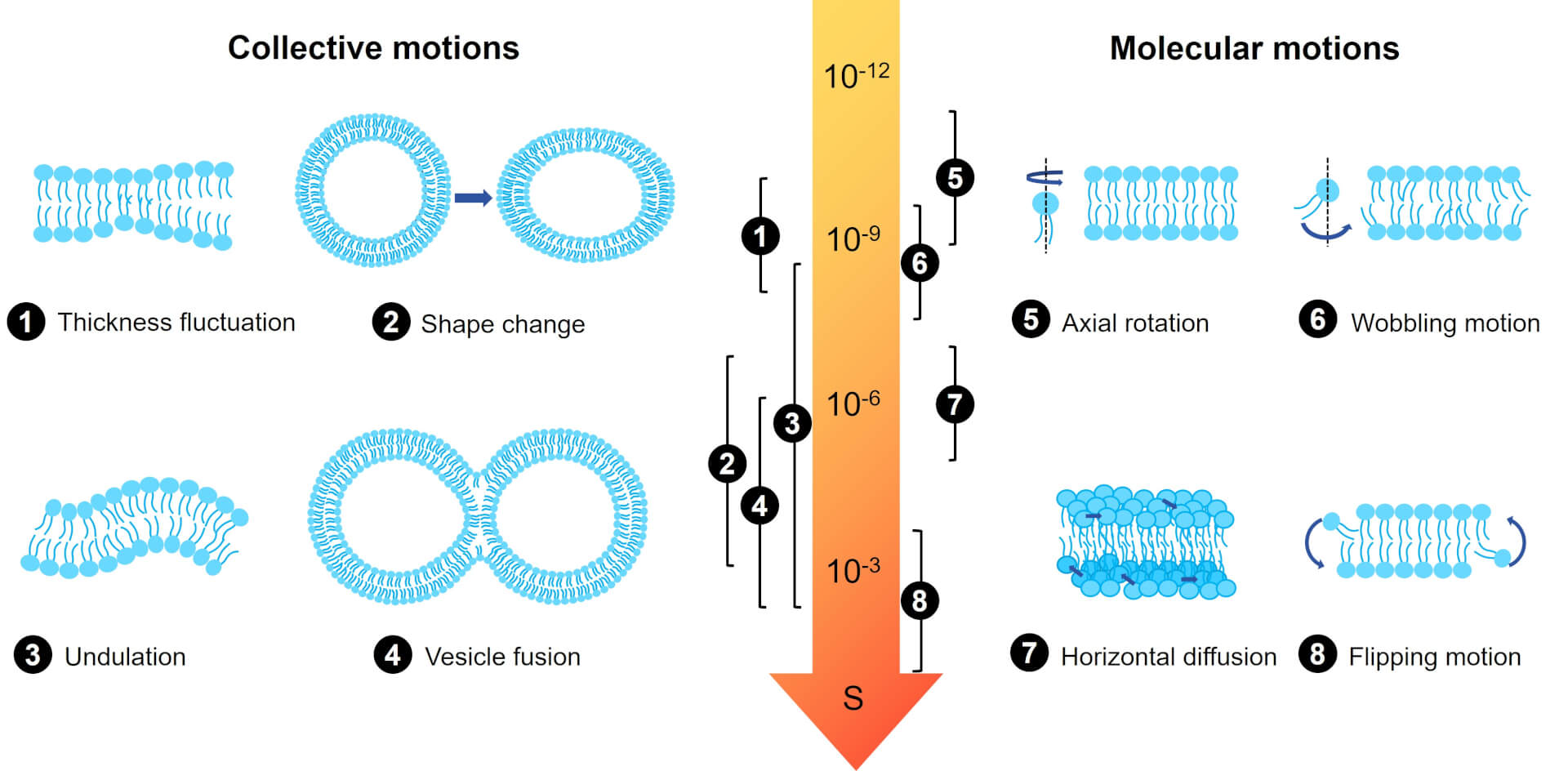 Fig.3 Different dynamics of lipid layer on different time scales.
Fig.3 Different dynamics of lipid layer on different time scales.
Characterization of Composition
The special problems in compositional analysis are the imbalance of lipid ratios in the layer and the detectability of degradation products from hydrolysis (e.g., fatty acids and lysophospholipids), particularly in the low molar range. We offer a wide range of analytical techniques to suit your needs:
- Laser sensing, time resolution, and long-wavelength fluorescence based on luminescence monitoring.
- High-performance liquid chromatography (HPLC) and mass spectrometry (MS) based on highly sensitive detection systems.
- Catalytic biosensors, immunosensors, and genetic sensors based on affinity.
- The screening systems of DNA probes and biochips are based on lab-on-chip technology.

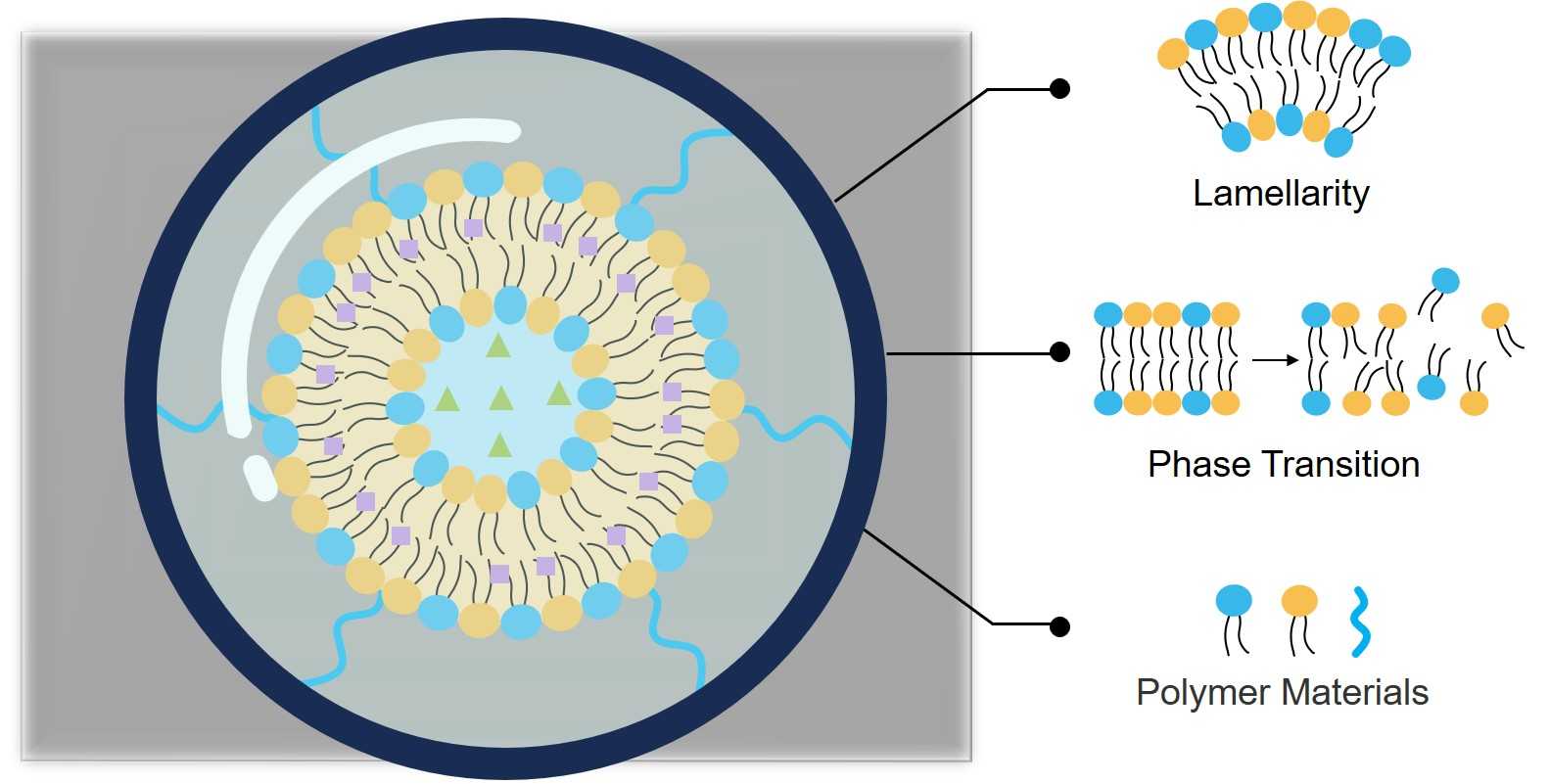 Fig 1. The structure and composition analysis of liposome formulations.
Fig 1. The structure and composition analysis of liposome formulations.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical Use