As a kind of artificially prepared vesicles, liposomes have become an important tool to improve the release of antibiotics, anticancer drugs, antifungal drugs, peptide hormones, enzymes, vaccines, and genetic materials. Due to different preparation methods and lipid composition, liposomes can be classified according to their lamellarity, particle size, charge, and application. Regardless of their solubility, the flexibility of their behavior can be utilized through different routes of administration.
The main purpose of ideal liposome preparation is to obtain high drug entrapment efficiency, narrow particle size distribution and long-term stability of liposome products. Therefore, generally speaking, the following aspects are involved in the design and preparation of liposomes:
Liposomes are small spherical vesicles composed of one or more lipid bilayers with an aqueous core. The preparation process can be divided into the following three basic steps:
When the lipid is dispersed in the aqueous medium by stirring, the formation of population vesicles may reach the size range. Liposomes can be prepared by mechanical methods, solvent dispersion methods and methods based on fusion or size conversion.
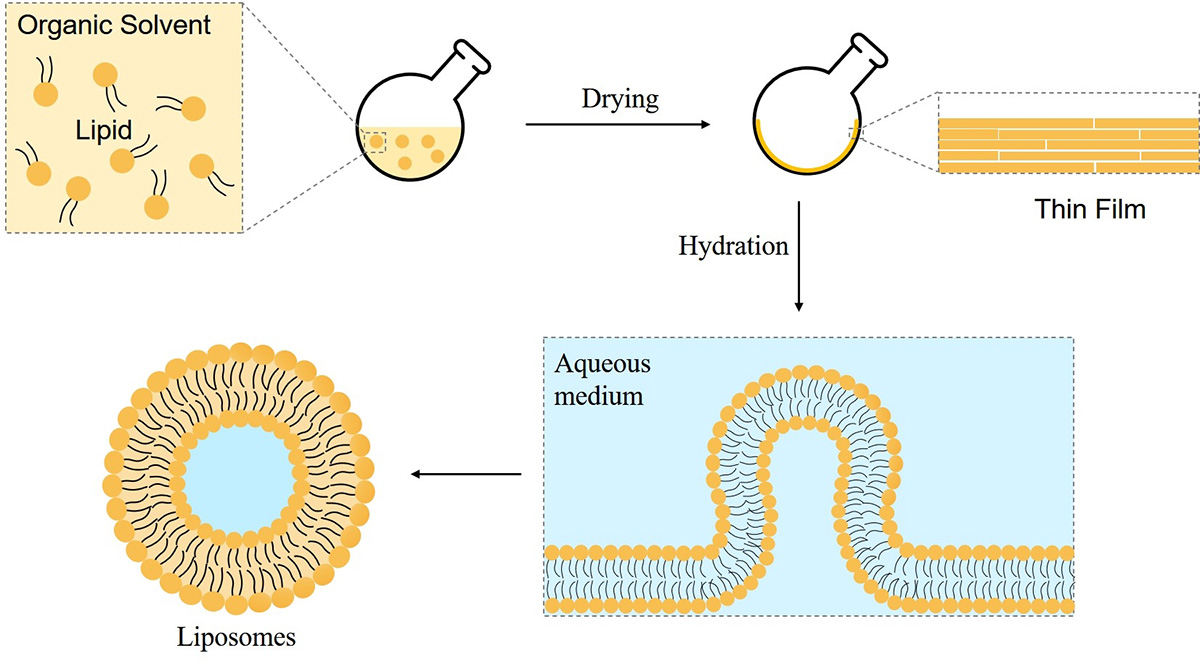 Fig.1 Preparation of Liposomes.
Fig.1 Preparation of Liposomes.
Many liposome drug products are designed to improve the stability of encapsulated active substances in vivo, the pharmacokinetics of active substances (including tissue distribution curve) and the intracellular behavior of active substances. Therefore, after determining the preparation method of liposomes, the determination of its dosage form is also very important, which can affect the safe and effective dosage and dosing schedule of liposome pharmaceutical preparations.
In practical application, emulsion is the main dosage form of liposome, but the long-term stability of this dosage form is poor, so it is not suitable for long-term storage. Lyophilization of liposomes into solid dosage forms provides the possibility of simple administration and shows higher storage stability than other dosage forms.
In order to ensure the quality of liposome drug products, not only the intermediate control and process control should be implemented in the production process, the quality of the final product should be tested, but also the appropriate control strategy should be formulated based on the understanding of the production process.
The quality control of liposomes is mainly evaluated from the aspects of particle size distribution, average particle size, surface morphology, entrapment efficiency, drug loading, stability, oxidation degree of phospholipids, phase transition temperature, residual amount of organic solvent and sterilization effect.
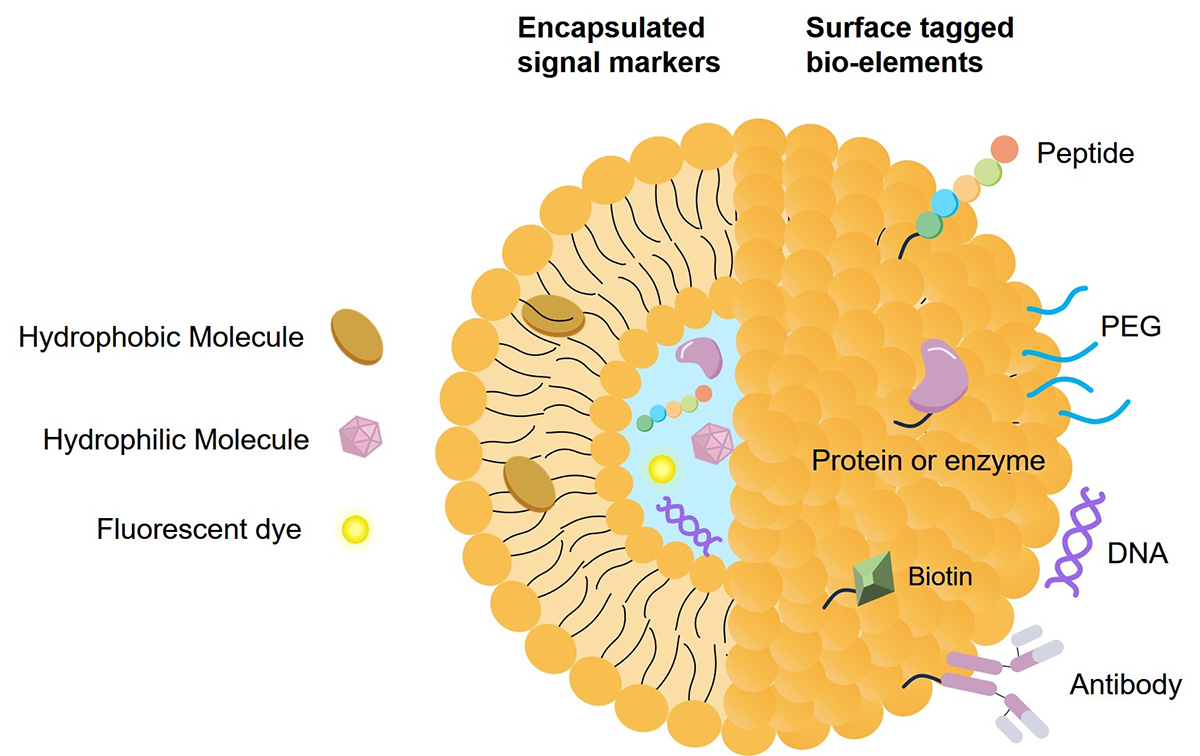 Fig.2 Structure and applications of liposomes.
Fig.2 Structure and applications of liposomes.
In the past few years, Creative Biolabs has made great progress in the design and production of liposome nanoparticles. Our service enables each liposome to have a unique specification and be subject to strict quality control and can ensure the quality and efficacy of liposomes during storage. For more details about our services, please contact us directly.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry