The term "liposome" was coined in the 1960s when it was discovered that sealed lipid bilayer vesicles could spontaneously form in water. However, the use of the phrase "lipid nanoparticles (LNPs)" came much later, around the early 1990s, with the dawn of the era of nanotechnology. Given that liposomes are made of lipids and are often of a nanosized dimension, they were naturally considered the first generation of lipid nanoparticles. In the pharmaceutical industry, "LNPs" have emerged as promising carriers for the delivery of various therapeutic drugs.
 Fig 1 COVID-19 Vaccine
Fig 1 COVID-19 Vaccine
A proposed technique for some infectious diseases, such as COVID-19, involves introducing a small fragment of the so-called coronavirus spike protein in the form of mRNA into the human body. This introduction could provoke an immune reaction against the expressed protein, thereby killing or deactivating invading coronaviruses. However, it was found that mRNA is too fragile to survive long enough in the body upon injection to produce the spike protein and elicit an immune response. Interestingly, tiny liposomes could solve this problem. The potential of liposomes as a drug delivery system was almost immediately recognized upon their discovery. Advantages of using liposomes to deliver mRNA into cells include:
LNPs feature prominently in the BNT162b2 vaccine and mRNA-1273 vaccine, acting as a delivery vehicle. These COVID-19 mRNA vaccines demonstrated notable efficacy in disease prevention. The LNPs in both vaccines are structurally akin. An ionizable lipid is included, which bears a positive charge at low pH facilitating RNA complexation, and is neutral at physiological pH, minimizing potential toxicity and encouraging payload discharge. A PEGylated lipid is also present, which reduces antibody attachment and phagocyte clearance, resulting in longer systemic circulation. The phospholipid DSPC and cholesterol aid in packing the cargo into the LNPs.
Lipid Components of COVID-19 LNPs-based mRNA Vaccines
| Vaccine Type | Lipid Name | Role | CAS Number | Price |
| BNT162b2 vaccine | ALC-0315 | ionizable cationic lipid | 2036272-55-4 | Inquiry |
| ALC-0159 | PEG-lipid | 1849616-42-7 | Inquiry | |
| DSPC | helper lipid | 816-94-4 | Inquiry | |
| Cholesterol | helper lipid | 57-88-5 | Inquiry | |
| mRNA-1273 vaccine | SM-102 | ionizable cationic lipid | 2089251-47-6 | |
| DMG-PEG 2000 | PEG-lipid | 160743-62-4 | Inquiry | |
| DSPC | helper lipid | 816-94-4 | Inquiry | |
| Cholesterol | helper lipid | 57-88-5 | Inquiry |
mRNA vaccines and therapies have vast potential in preventing and treating diseases. LNP-based intracellular delivery of mRNA allows for the expression of any desired protein in host cells. A key characteristic of mRNA-based therapeutics is their lower risk of induced mutation. As mRNA does not integrate into the host genome, mRNA-based therapies have reduced carcinogenic and mutagenic risks, amplifying their safety profile. Furthermore, producing mRNA is easier to standardize than DNA, and it offers superior reproducibility.
mRNA vaccines have revolutionized vaccine development due to their high efficiency, accelerated production cycle, and the potential for low-cost manufacturing. The rapid advancement of mRNA vaccines would not have been possible without progressing LNP nucleic acid delivery technology. LNPs are integral for successfully delivering mRNA to the cytosol of immune cells within cells, particularly the antigen-presenting immune cells responsible for triggering the desired immune response. LNP-based mRNA vaccines have entered clinical trials for multiple infectious diseases, such as vaccines against the Zika virus, cytomegalovirus, tuberculosis, and modified nucleoside mRNA influenza. mRNA therapeutic vaccines also have vast potential in cancer immunotherapy against melanoma, ovarian cancer, breast cancer, and other solid tumors. The expected impact of this innovative mRNA-based therapeutic and vaccine paradigm is widespread.
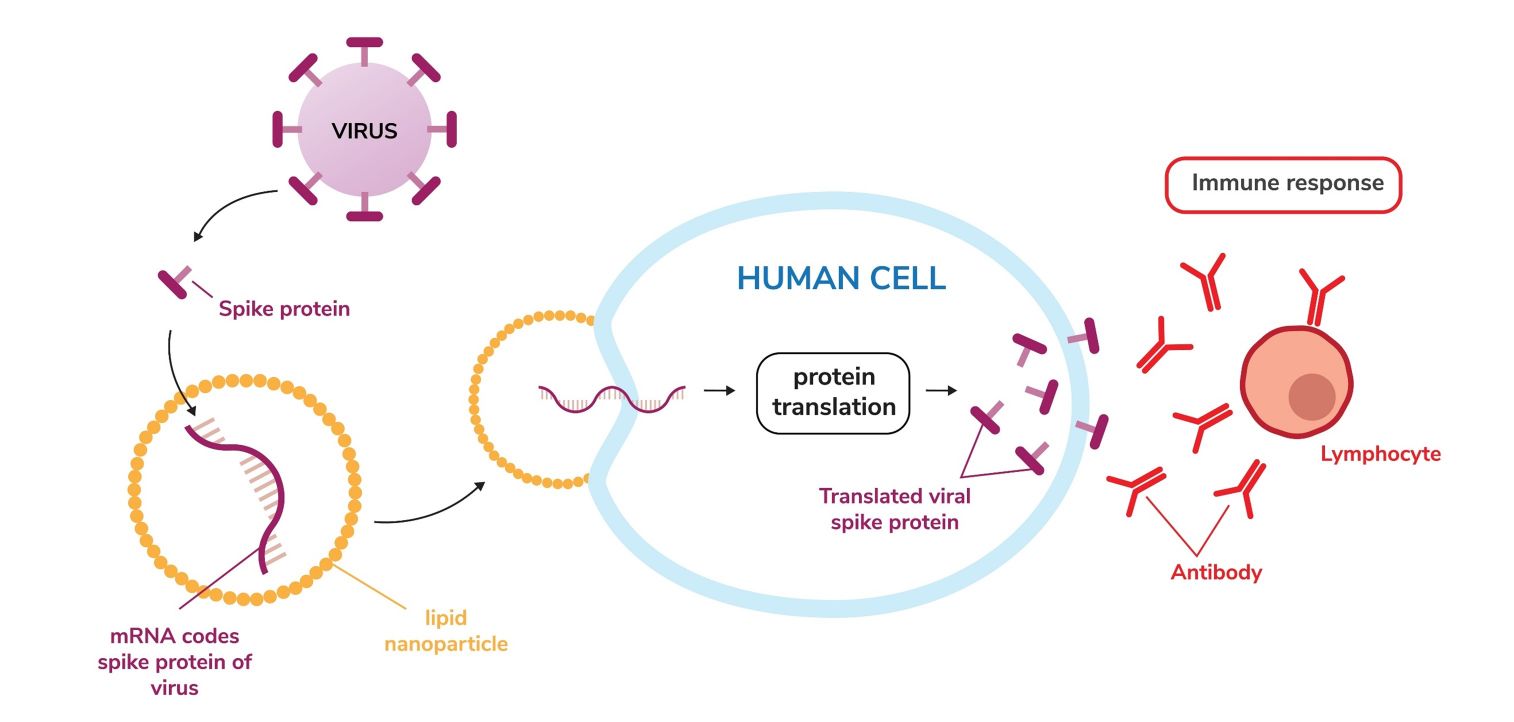 Fig 2 Mechanism of LNP-based mRNA Vaccine
Fig 2 Mechanism of LNP-based mRNA Vaccine
The process of developing LNP-based mRNA involves several stages. Initially, mRNA is synthesized in vitro using a DNA template, tailored to encode a specific protein, typically linked to disease. Subsequently, the mRNA is enclosed within an LNP, offering protection against degradation, and facilitating cell entry. Formation of LNPs involves merging lipids in an organic solvent, then swiftly mixing with an aqueous solution containing the mRNA, creating nanoparticles. The blend then undergoes purification to eliminate any unmatched mRNA or residual organic solvents. Later, the formed LNPs, carrying the mRNA, are assessed for size, encapsulation proficiency, and potency. The final nano-formulation is then subjected to stringent preclinical and clinical trials to confirm safety, effectiveness, dosage, and delivery techniques. Creative Biolabs provides one-stop customized services to aid your LNPs-based mRNA vaccine development.
| Services | Features | Price |
| Functional Liposome Development | We can rationally design and develop different liposomes according to client needs. | Inquiry |
| Liposome Encapsulated Nucleic Acid | Our well-designed liposomal delivery system can reduce the toxicity and increase the efficacy of NA-based drugs. | Inquiry |
| LNP Development | Our services are comprehensive including LNP formulation screening and optimization, functional verification, scale-up, and process optimization, etc. | Inquiry |
| Liposome Analysis and Characterization | We have extensive experience in supporting liposomal formulation characterization, release testing, and stability program management. | Inquiry |
LNP-mRNA vaccines' administration routes are primarily bifurcated into two strategies: systemic administration and localized administration.
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry