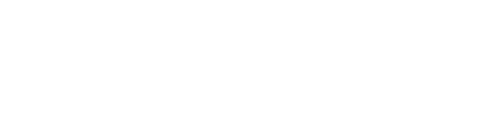Sonication Technique
Sonication is a simple method for reducing the size of liposomes and manufacture of nanoliposomes. The common laboratory method involves treating hydrated vesicles for several minutes with a titanium-tipped probe sonicator in a temperature controlled environment.
- Dissolve a suitable combination of the phospholipid components, with or without cholesterol, in either chloroform or in chloroform–methanol mixture (usually 2:1 v/v).
- Filter the mixture to remove minor insoluble components or ultrafilter to reduce or eliminate pyrogens.
- Transfer the solution to a pear-shaped or a round-bottom flask and, employing a rotary evaporator, remove the solvents at temperatures above Tc under negative pressure, leaving a thin layer of dried lipid components in the flask. Other methods of drying the lipid ingredients include lyophilization and spray drying.
- Remove traces of the organic solvents using a vacuum pump, usually overnight at pressures below 0.1 Pa. Alternatively, traces of the organic solvents may be removed by flushing the flask with an inert gas, such as nitrogen or argon.
- After drying the lipid ingredients, small quantity of glass beads (e.g., with 500 mm diameter) are added to the flask containing the dried lipids following by the addition of a suitable aqueous phase such as distilled water or buffer. Alternative hydration mediums are saline or nonelectrolytes such as a sugar solution. For an in vivo preparation, physiological osmolality (290 mOsmol/kg) is recommended and can be achieved using 0.6% saline, 5% dextrose, or 10% sucrose solution. The aqueous medium can contain salts, chelating agents, stabilizers, cryoprotectants (e.g., glycerol) and the drug to be entrapped.
- The dried lipids can be dispersed into the hydration fluid by hand shaking the flask or vortex mixing for 1–5 min. At this stage, micrometric MLV type liposomes are formed.
-
Transfer the flask containing MLV either to a bath-type sonicator or a probe (tip) sonicator. For probe sonication, place the tip of the sonicator in the MLV flask and sonicate the sample with 20 s ON, 20 s OFF intervals (to avoid over-heating), for a
total period of 10–15 min. At this stage, nanoliposomes are formed, which are predominantly in the form of small unilamellar vesicles (SUV). Alternatively, nanoliposomes can be produced using a bath sonicator as explained in the following
section.
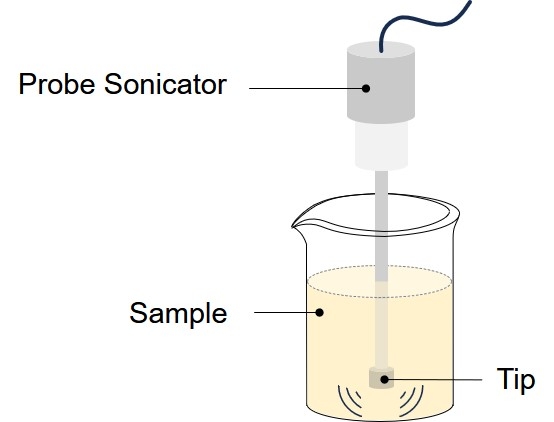 Fig.1 Schematic representation of a probe-type sonicator.
Fig.1 Schematic representation of a probe-type sonicator.
- Fill the bath sonicator with room temperature water mixed with a couple of drops of liquid detergent. Using a ring stand and test tube clamp, suspend the MLV flask in the bath sonicator. The liquid level inside the flask should be equal to that of outside the flask. Sonicate for a time period of 20–40 min.
- Store the final product at temperatures above Tc under an inert atmosphere such as nitrogen or argon for 1 h to allow the annealing process to come to completion. Mean size and polydispersity index of vesicles is influenced by lipid composition and concentration, temperature, sonication time and power, sample volume, and sonicator tuning. Since sonication process is difficult to reproduce, size variation between batches produced at different times is not uncommon.
- Residual large particles remaining in the sample can be removed by centrifugation to yield a clear suspension of nanoliposomes.
Extrusion Method
Extrusion is a process by which micrometric liposomes (e.g., MLV) are structurally modified to large unilamellar vesicles (LUV) or nanoliposomes depending on the pore-size of the filters used. Vesicles are physically extruded under pressure through polycarbonate filters of defined pore sizes. A protocol for using a small-sized extruder is described in the following section. A mini extruder device, with 0.5 mL or 1 mL gas-tight syringes can be employed in this procedure.
- Prepare a liposome sample, such as MLV, as explained earlier.
- Place one or two-stacked polycarbonate filters into the stainless steel filter-holder of the extruder.
- Place the extruder stand/heating block onto a hot plate. Insert a thermometer into the well provided in the heating block. Switch the hot plate on and allow the temperature to reach a temperature above Tc of the lipids.
-
In order to reduce the dead volume, pre-wet the extruder parts by passing a syringe full of buffer through the extruder and then discard the buffer.
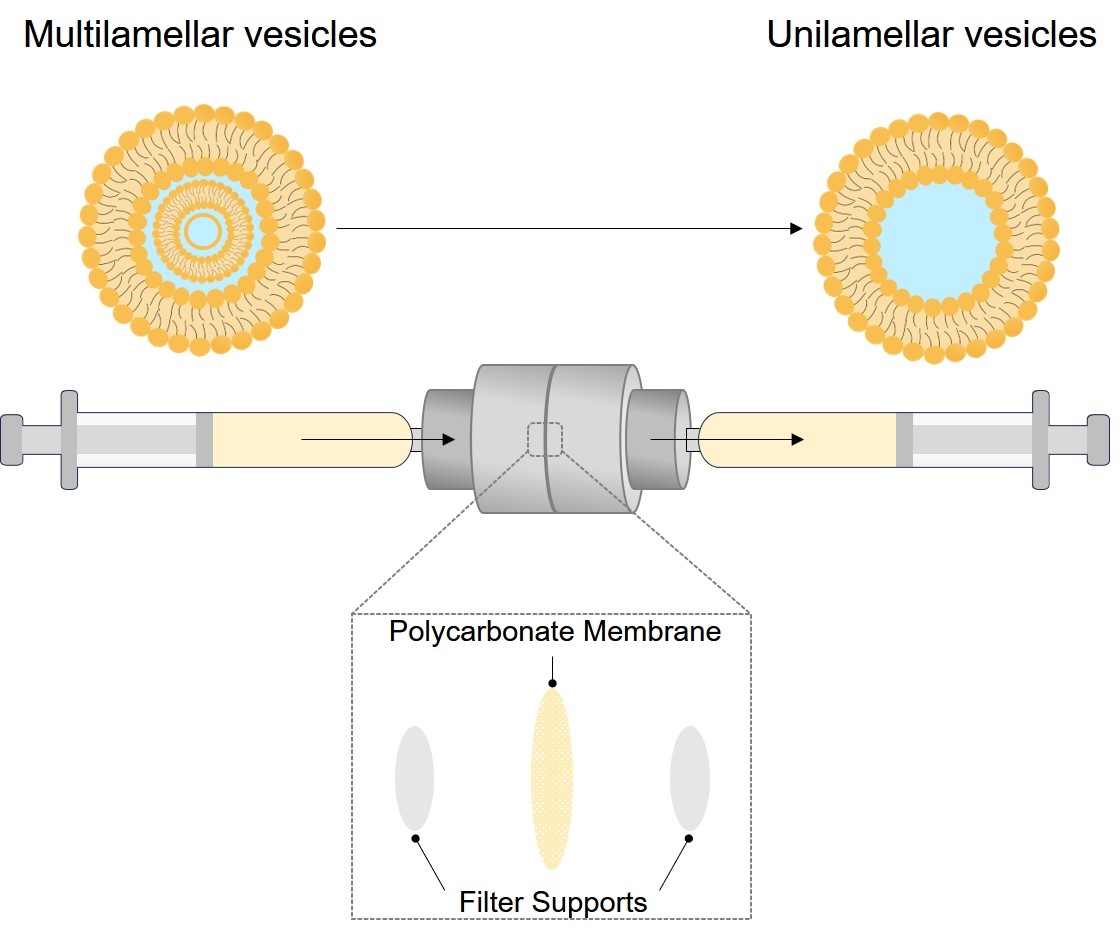 Fig.2 A small, hand-held, extruder used in the manufacture of nanoliposomes.
Fig.2 A small, hand-held, extruder used in the manufacture of nanoliposomes.
- Load the liposome suspension into one of the syringes (donor syringe) of the mini extruder and carefully place the syringe into one end of the extruder by applying a gentle twisting.
- Place the second syringe (receiver syringe) into the other end of the extruder. Make sure the receiver syringe plunger is set to zero.
- Insert the fully assembled extruder device into the extruder stand. Insert the stainless-steel hexagonal nut in such a way that any two opposing apexes fall in the vertical plane. Use the swing-arm clips to hold the syringes in good thermal contact with the heating block.
- Allow the temperature of the liposome suspension to reach the temperature of the heating block (approximately 5–10 min).
- Gently push the plunger of the filled syringe until the liposome suspension is completely transferred to the empty syringe.
- Gently push the plunger of the alternate syringe to transfer the suspension back to the original syringe.
- Repeat the extrusion process for a minimum of seven passes through the filters. In general, the more passes though the filters, the more homogenous the sample becomes. In order to reduce the possibility of sample contamination with larger particles or foreign material, the final extrusion should fill the receiver syringe. Therefore, an odd number of passages through the filters should be performed.
- Carefully remove the extruder from the heating block. Remove the filled syringe from the extruder and inject the nanoliposome sample into a clean vial.
- The extruder components can be cleaned by first rinsing with ethanol (or leaving the extruder parts in warm 70% ethanol for few hours) and then rinsing with distilled water.
- Keep the final product at temperatures above Tc under an inert atmosphere such as nitrogen or argon for 1 h to allow the sample to anneal and stabilize.
Microfluidization
A method of nanoliposome production without using potentially toxic solvents is the microfluidization technique using a microfluidizer. Figure 3 shows a schematic representation of a microfluidizer devices. This apparatus has been traditionally used in the pharmaceutical industry to make liposomal products and pharmaceutical emulsions. Microfluidization is based on the principle of dividing a pressure stream into two parts, passing each part through a fine orifice, and directing the flows at each other inside the chamber of microfluidizer. Within the interaction chamber, cavitation, along with shear and impact, reduces particle sizes of the liposomes. Microfluidizer uses high pressures (up to l0,000 psi) to guide the flow stream through microchannels toward the impingement area. The advantages of microfluidization are that: a large volume of liposomes can be formed in a continuous and reproducible manner; the average size of the liposomes can be adjusted; very high capture efficiencies (>75%) can be obtained; and the solutes to be encapsulated are not exposed to sonication, detergents or organic solvents. The process involves only a few steps as exemplified in the following section.
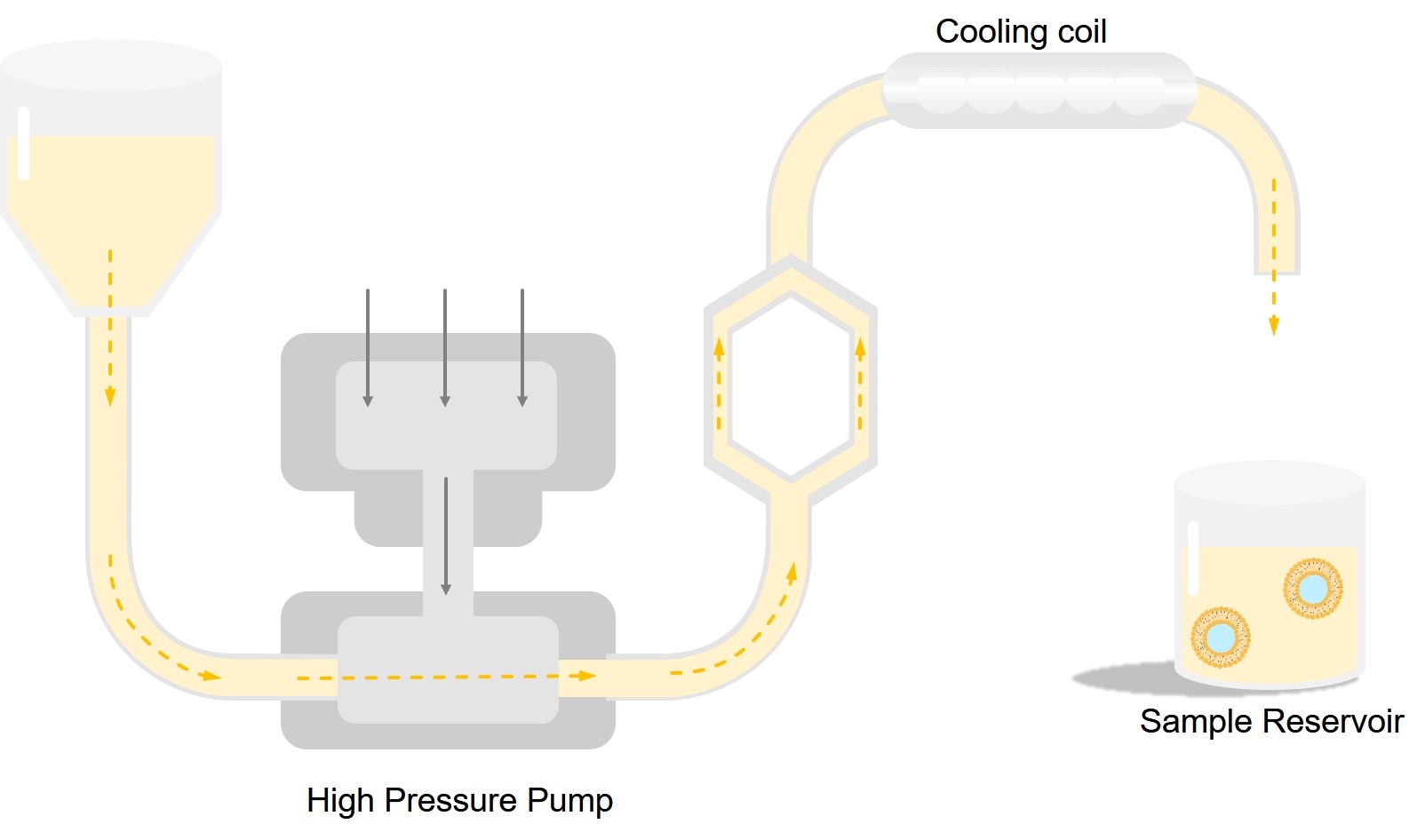 Fig.3 Main components of a microfluidizer device.
Fig.3 Main components of a microfluidizer device.
- Select the ingredients of the nanoliposomes and their suspension medium based on the intended application. The suspension medium is usually an aqueous phase such as deionized/distilled water or buffer. Alternative hydration mediums are saline or nonelectrolytes such as a sugar solution as explained earlier.
- Prepare a phospholipid dispersion by placing the nanoliposomal ingredients in the suspension medium and then mixing the sample by stirring e.g. employing a blender or by using a homogenizer.
- Pass the dispersion through the microfluidizer by placing the crude suspension of phospholipids in the reservoir and adjusting the air regulator to the selected operating pressure. With an optimized setting, when the air valve is open, the liquid dispersion flows through a filter into the interaction chamber where it is separated into two streams that interact at extremely high velocities in dimensionally defined microchannels.
- The suspension can be recycled through the equipment in which case the suspension must be cooled because of the temperature increase in the interaction chamber at high operating pressure.
- After sample collection, the microfluidizer can be cleaned by recycling 95% ethanol followed by passing distilled water through the system.
- Leave the nanoliposome suspension at temperatures above Tc under an inert atmosphere such as nitrogen or argon for 1 h to allow the sample to anneal and stabilize.
Heating Method
Majority of nanoliposome manufacture techniques either involve utilization of potentially toxic solvents (e.g. chloroform, methanol, diethyl ether and acetone) or high shear force procedures. It has been postulated that residues of these toxic solvents may remain in the final liposome or nanoliposome preparation and contribute to potential toxicity and influence the stability of the lipid vesicles. Although there are methods to decrease the concentration of the residual solvents in liposomes (e.g. gel filtration, dialysis and vacuum), these are practically difficult and time-consuming procedures. In addition, the level of these solvents in the final formulations must be assessed to ensure the clinical suitability of the products. Therefore, it would be much preferable to avoid the utilization of these solvents in nanoliposome manufacture, which will also bring down the time and cost of preparation especially at the industrial scales.
Regarding the utilization of high pressures or high shear forces during nanoliposome manufacture (e.g. as occurs during microfluidization), there are reports on the deleterious effects of these procedures on the structure of the material to be encapsulated. These hurdles can be overcome by employing alternative preparation methods such as the heating method by which liposomes and nanoliposomes (in addition to some other carrier systems) can be prepared using a single apparatus in the absence of potentially toxic solvents.
- Hydrate a suitable combination of the phospholipid components, with or without cholesterol in an aqueous medium for a time period of 1–2 h under an inert atmosphere such as nitrogen or argon. The nanoliposomal ingredients may be hydrated together or separately based on their solubility and Tc.
- Mix the lipid dispersions along with the material to be encapsulated, in a heat-resistant flask such as a pyrex beaker, and add glycerol to a final volume concentration of 3%. Alternatively a heat-resistant bottle with six baffles can be used for the process as explained and pictured in reference
- For the preparation of cholesterol-containing formulations, first dissolve cholesterol in the aqueous phase at elevated temperatures (e.g. 120°C) while stirring (approx. 1,000 rpm) for a period of 15–30 min under nitrogen atmosphere before adding the other components as mentioned earlier.
- Depending on the nanoliposomal ingredients, sample volume, type of flask used and its number of baffles, as well as type, speed and duration of mixing, nanovesicles can be produced without the need to perform filtration or sonication.
- Leave the nanoliposome suspension at temperatures above Tc under an inert atmosphere such as nitrogen or argon for 1 h to allow the sample to anneal and stabilize.
Liposomes and nanoliposomes prepared by the heating method (HM-liposomes) have been employed successfully as gene transfer vectors as well as drug delivery vehicles. Incorporation of plasmid DNA molecules, which are sensitive to high temperatures, to the HM-liposomes was carried out at room temperature by incubation of DNA with the empty, pre-formed, HM-liposomes. Another important feature of this method is that it can be easily adapted from small to industrial scales. Incorporation of drugs into the HM-liposomes can be achieved by several routes including:
- Adding the drug to the reaction medium along with the liposomal ingredients and glycerol.
- Adding the drug to the reaction medium when temperature has dropped to a point not lower than the transition temperature (Tc) of the lipids.
- Adding the drug to the HM-liposomes after the vesicles are prepared e.g. at room temperature (incorporation of DNA to the HM-liposomes was performed by this route as explained earlier).
Therefore, the heating method has flexibility for the entrapment of various drugs and other active substances with respect to their temperature sensitivities. Recently, Mozafari and colleagues showed that nanoliposomes prepared by the heating method are completely non-toxic towards cultured cells while nanoliposomes prepared by a conventional method using volatile solvents revealed significant levels of cytotoxicity.
Mozafari Method
A further improved version of the heating method, called Mozafari method, is one of the most recently introduced and one of the most simple techniques for the preparation of liposomes and nanoliposomes (in addition to some other carrier systems). The Mozafari method has recently been employed successfully for the encapsulation and targeted delivery of the food-grade antimicrobial nisin. The Mozafari method allows manufacture of carrier systems in one-step, without the need for the pre-hydration of ingredient material, and without employing toxic solvents or detergents from small scales to large, industrial scales. The mentioned method is economical and capable of manufacturing nanoliposomes, with a superior monodispersity and storage stability using a simple protocol and one, single vessel. Encapsulation of nisin (as an example of a substance with low water solubility) in nanoliposomes prepared by the Mozafari method is explained in the following section.
- Add the liposomal ingredients to a preheated (60°C, 5 min) mixture of nisin (200 μg/ml) and a polyol such as glycerol (final concentration 3%, v/v) in a heat-resistant flask such as a pyrex beaker. Alternatively, a heat-resistant bottle with six baffles can be used as explained and pictured in reference.
- Heat the mixture at 60°C while stirring (approx. 1,000 rpm) on a hotplate stirrer for a period of 45–60 min under nitrogen atmosphere.
- For the preparation of cholesterol-containing formulations, first dissolve the cholesterol in the aqueous phase at elevated temperatures (c. 120°C) while stirring (approx. 1,000 rpm) for a period of 15–30 min under nitrogen atmosphere before adding the other phospholipid components.
- Depending on the formulation ingredients, sample volume, type of flask used and its number of baffles, as well as type, speed and duration of mixing, nanometric vesicles can be produced in one step, without the need to perform filtration or sonication.
- Leave the nanoliposome suspension at temperatures above Tc c under an inert atmosphere such as nitrogen or argon for 1 h to allow the sample to anneal and stabilize.
Analysis Methods
Characterization and Analysis of Nanoliposomes
Following preparation of nanoliposomes, especially when using a new technique, characterization is required to ensure adequate quality of the product. Methods of characterization must be meaningful and preferably rapid. Several techniques such as electron microscopy, radiotracers, fluorescence quenching, ultrasonic absorption, electron spin resonance spectroscopy, and nuclear magnetic resonance spectroscopy may be used to characterize nanoliposome formulations. Each technique has characteristic advantages and possible disadvantages. The most important parameters of nanoliposome characterization include visual appearance, size distribution, stability, Zeta potential, lamellarity and entrapment efficiency.
- Visualization Techniques. Many imaging techniques are available for visualization of nanoliposomes. An optical microscope (phase contrast) can detect particles larger than 300 nm and contamination with larger particles. A polarizing microscope can reveal lamellarity of liposomes. For instance, MLV-type liposomes are birefringent and display a Maltese cross. The size distribution of nanoliposomes is mainly determined using electron microscopy. Negative staining, freeze-fracture and scanning electron microscopy are the methods most commonly used to characterize nanoliposome structures. A more recently developed microscopic technique with very high resolutions is the Scanning Probe Microscopy (SPM). Two of the most applied SPM techniques are Scanning Tunnelling Microscopy (STM) and Atomic Force Microscopy (AFM). This recent technology gives the possibility to view various biological and non-biological samples under air or water with a resolution up to 3A. By this method, monolayers of various lipids and lipid-attached molecules such as antibody fragments can be studied.
- Size Determination. Size and size distribution (polydispersity) of the formulated nanoliposomes are of particular importance in their characterization. Maintaining a constant size and/or size distribution for a prolonged period is an indication of liposome stability. Electron microscopic methods are widely used for establishing the morphology, size and stability of liposomes. With respect to a statistically meaningful analysis of size distribution of the lipid vesicles, methods such as light scattering, which measure the size of many vesicles in an aqueous medium, are more appropriate than microscopic techniques. Ideally, these two techniques need to be employed along with other inexpensive and routine laboratory techniques, such as gel permeation chromatography, to provide a comprehensive and reliable characterisation of the nanoliposomal formulations.
- Each of the currently used particle size determination techniques has its own advantages and disadvantages. Light scattering, for example, provides cumulative average information of the size of many nanoliposomes simultaneously. However, it does not provide information on the shape of the lipidic system and it assumes any aggregation of more than one vesicle as one single particle. Electron microscopic techniques, on the other hand, make direct observation possible; hence provide information on the shape of the vesicles as well as presence/absence of any aggregation and/ or fusion. The drawback of the microscopic investigations is that the number of particles that can be studied at any certain time is limited. Therefore, the general approach for the determination of size distribution of nanoliposomal formulations should be to use as many different techniques as possible.
- Zeta Potential. The other important parameter in liposome characterization is zeta potential. Zeta potential is the overall charge a lipid vesicle acquires in a particular medium. It is a measure of the magnitude of repulsion or attraction between particles in general and lipid vesicles in particular. Evaluation of the zeta potential of a nanoliposome preparation can help to predict the stability and in vivo fate of liposomes. Any modification of the nanoliposome surface, e.g. surface covering by polymer(s) to extend blood circulation life, can also be monitored by measurement of the zeta potential. Generally, particle size and zeta potential are the two most important properties that determine the fate of intravenously injected liposomes. Knowledge of the zeta potential is also useful in controlling the aggregation, fusion and precipitation of nanoliposomes, which are important factors affecting the stability of nanoliposomal formulations.
- Lamellarity Determination. The lamellarity of liposomes made from different ingredients or preparation techniques varies widely. This is evidenced by reports showing that the fraction of phospholipid exposed to the external medium has ranged from 5% for large MLV to 70% for SUV. Liposome lamellarity determination is often accomplished by 31P NMR. In this technique, the addition of Mn2+ quenches the 31P NMR signal from phospholipids on the exterior face of the liposomes and nanoliposomes. Mn2+ interacts with the negatively charged phosphate groups of phospholipids and causes a broadening and reduction of the quantifiable signal. The degree of lamellarity is determined from the signal ratio before and after Mn2+ addition. While frequently used, this technique has recently been found to be quite sensitive to the Mn2+ and buffer concentration and the types of liposomes under analysis. Other techniques for lamellarity determination include electron microscopy, small angle X-ray scattering (SAXS), and methods that are based on the change in the visible or fluorescence signal of marker lipids upon the addition of reagents.
-
Encapsulation Efficiency. Encapsulation efficiency is commonly measured by encapsulating a hydrophilic marker (i.e. radioactive sugar, ion, fluorescent dye), sometimes using single-molecule detection. The techniques used
for this quantification depend on the nature of the entrapped material and include spectrophotometry, fluorescence spectroscopy, enzyme-based methods, and electrochemical techniques.
If a separation technique such as HPLC or FFF is applied, the percent entrapment can be expressed as the ratio of the unencapsulated peak area to that of a reference standard of the same initial concentration. This method can be applied if the nanoliposomes do not undergo any purification following the preparation. Any of the purification technique serves to separate nanoliposome-encapsulated materials from those that remain in the suspension medium. Therefore, they can also be used to monitor the storage stability of nanoliposomes in terms of leakage or the effect of various disruptive conditions on the retention of encapsulants. In the latter case, total lysis can be induced by the addition of a surfactant such as Triton X100. Retention and leakage of the encapsulated material depend on the type of the vesicles, their lipid composition and Tc, among other parameters. It has been reported that SUV and MLV-type liposomes are less sensitive than LUV liposomes to temperature-induced leakage. This property of liposomes and nanoliposomes can be used in the formulation of temperature-sensitive vesicles.
Since techniques used to separate nanoliposome-entrapped from free material can potentially cause leakage of contents and in some cases, ambiguity in the extent of separation, research using methods that do not rely on separation are of interest. Reported methods include 1 H NMR where free markers exhibit pH sensitive resonance shifts in the external medium versus encapsulated markers; diffusion ordered 2D NMR, which relies on the differences in diffusion coefficients of entrapped and free marker molecules; fluorescence methods where the signal from unencapsulated fluorophores was quenched by substances present in the external solution; and electron spin resonance (ESR) methods which rely on the signal broadening of unencapsulated markers by the addition of a membrane-impermeable agent.

 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical Use