Cellulose-Based Delivery Systems: A Complete, Simple Guide for Modern Drug Delivery
Cellulose-based drug delivery systems are gaining rapid attention because they offer a simple, safe, and highly adaptable way to control how drugs are carried and released in the body. As more researchers look for biocompatible and sustainable materials, cellulose and its derivatives—ranging from nanocellulose to advanced hydrogels—are becoming essential tools across modern formulation strategies. In this article, Creative Biolabs will break down these systems in clear, practical terms, helping you understand how they work and why they matter in today's evolving drug delivery landscape.
What Are Cellulose-Based Drug Delivery Systems?
Definition
Cellulose is a natural polymer found mainly in plant walls. When it is purified and chemically modified, it becomes a powerful tool for drug delivery. In simple terms, cellulose-based drug delivery systems are platforms where cellulose or its derivatives are used to:
- Hold a drug in place
- Protect it from the environment
- Control how fast and where it is released
The cellulose-based drug delivery systems can be made from:
- Medical cellulose (high-purity cellulose used in medical products)
-
Cellulose derivatives, such as:
﹣ Microcrystalline cellulose (MCC)
﹣ Hydroxypropyl methylcellulose (HPMC)
﹣ Ethyl cellulose (EC)
﹣ Carboxymethyl cellulose (CMC)
﹣ Cellulose esters
Significance
They are attractive because they are:
- Biocompatible: generally well tolerated by the body
- Biodegradable: many grades can break down over time
- Tunable: by changing structure and chemistry, you can tune swelling, strength, and release rate
- Regulator: Many cellulose derivatives are already widely used as excipients
Today, cellulose-based delivery systems include nanocellulose particles, nanofibers, nanocrystals, hydrogels, and bacterial cellulose dressings, all of which open up new strategies for modern drug delivery.
Types of Cellulose Used in Drug Delivery
According to different sources or forms, there are five types of cellulose. Each type behaves differently and offers unique advantages, which is why researchers choose different celluloses for different delivery strategies.
Plant-Derived Cellulose (Native Cellulose)
- This type of cellulose is naturally found in plants such as wood, cotton, and agricultural fibers.
-
It is the base material that can be purified and turned into many useful forms.
- α-Cellulose – the purest structural fraction in plant fibers
- Microcrystalline Cellulose (MCC) – a purified, partially depolymerized form widely used in tablets
- Powdered Cellulose – cellulose fibers ground into fine particles
- Main use: tablets, capsules, fillers, binders, and matrix systems.
Cellulose Derivatives (Chemically Modified Cellulose)
- These forms are created by modifying cellulose chemically to improve solubility, swelling, or film-forming properties. They are the workhorse materials in pharmaceutical drug delivery.
-
Common types include:
- HPMC (Hydroxypropyl Methylcellulose)
- EC (Ethyl Cellulose)
- CMC (Carboxymethyl Cellulose)
- HPC (Hydroxypropyl Cellulose)
- Cellulose Acetate / Cellulose Esters
- Main use: controlled-release coatings, matrix tablets, gels, eye drops, suspensions.
Nanocellulose (Nanoscale Cellulose)
- Nanocellulose includes cellulose structures at the nanoscale, giving them high surface area, tunable chemistry, and strong mechanical properties.
-
Three main types (Figure 1):
- Cellulose Nanocrystals (CNCs) – rigid, rod-shaped nanoparticles
- Cellulose Nanofibers (CNFs) – long, flexible fibrils forming webs or networks
- Cellulose Nanoparticles (CNPs) – spherical nano-objects
- Main use: nanocarriers, scaffolds, transdermal films, advanced hydrogels, targeted delivery.
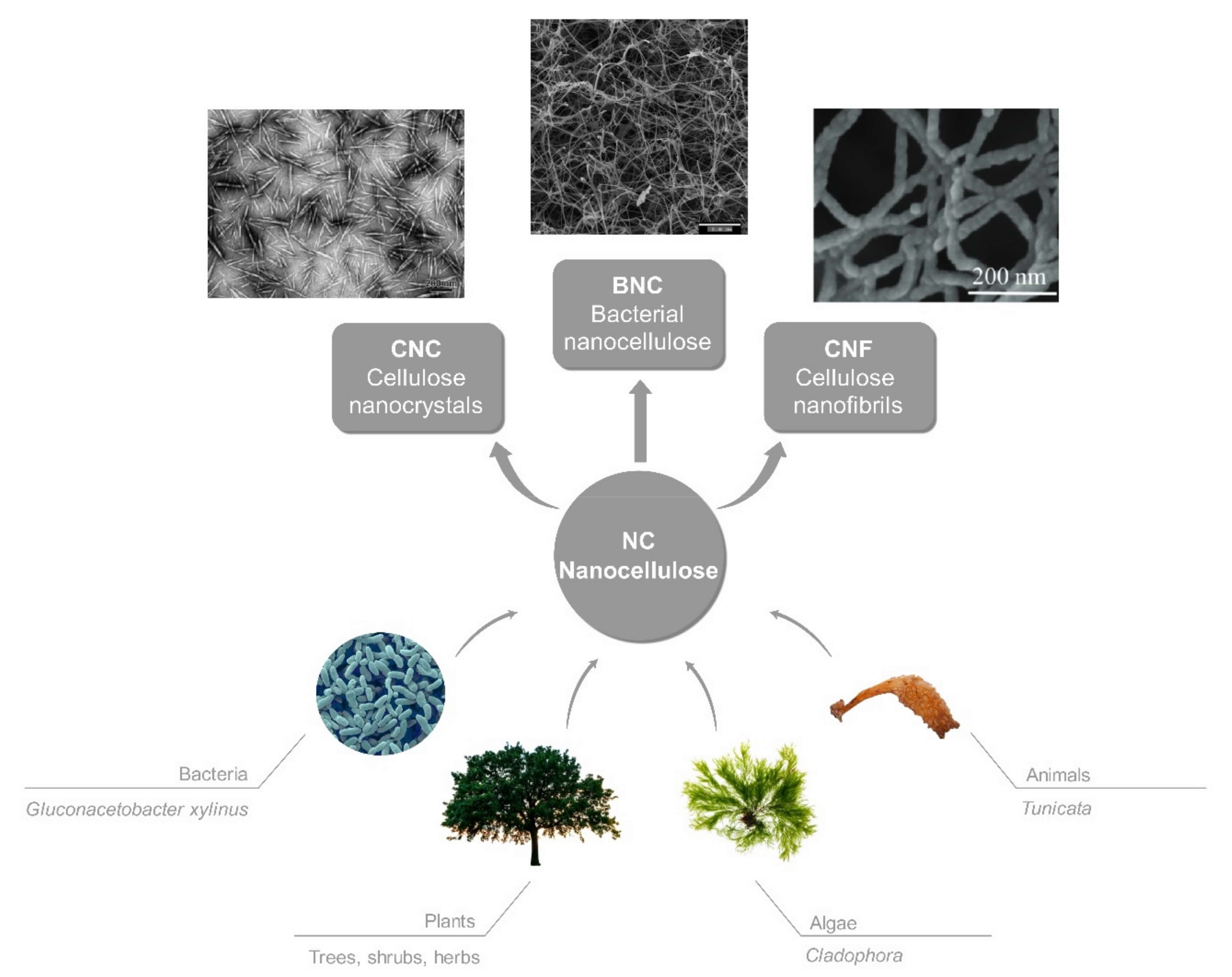 Fig.1
Different sources and types of nanocellulose.3
Fig.1
Different sources and types of nanocellulose.3
Bacterial Cellulose (Microbial Cellulose)
- Produced by bacteria rather than plants, bacterial cellulose is extremely pure and forms ultrafine nanofiber networks.
-
Key characteristics:
- No lignin, no hemicellulose
- High water content
- High crystallinity
- Excellent mechanical strength
- Main use: wound dressings, transdermal patches, local drug release membranes, scaffolds.
Cellulose-Based Hydrogels
- These are water-rich, three-dimensional networks made using cellulose or cellulose derivatives.
-
Types include:
- Pure cellulose hydrogels
- Cellulose-polymer hybrid hydrogels
- CNC-reinforced hydrogels
- Main use: topical gels, injectable depots, regenerative medicine scaffolds, slow-release implants.
To explore how these cellulose types can be engineered into targeted delivery platforms, you can also visit our comprehensive overview of modular delivery systems:
Creative Biolabs Targeted Delivery Module Systems
Applications of Key Cellulose-Based Delivery Systems
1. Nanocellulose Delivery Platforms
1.1 Nanocellulose-based delivery systems use cellulose in the nanoscale range to carry and release active ingredients. Because of their tiny size and large surface area, they can:
- Improve drug loading, even for poorly soluble drugs
- Offer precise control over release profiles
- Be functionalized with targeting moieties such as peptides, antibodies, or sugars
1.2 Key advantages include:
- Large surface area for high loading and strong interactions
- Tunable surface chemistry, thanks to plenty of hydroxyl groups
- Mechanical robustness, useful in scaffolds and films
- Compatibility with many routes, including oral, topical, transdermal, and implantable systems
1.3 For example:
- CNCs can be turned into nanocarriers for small molecules and biologics
- CNF-based mats can function as drug-releasing wound dressings or tissue scaffolds
When combined with targeted delivery strategies, nanocellulose becomes a smart platform for cellulose-based delivery strategies that can match specific tissues or disease sites.
2. Bacterial Cellulose for Wound Dressings & Local Delivery
2.1 Bacterial cellulose (BC) is produced by certain bacteria, not by plants. It forms a very pure, highly hydrated network of nanofibers. This structure is ideal for:
- Moist wound healing
- Oxygen transport
- Conformable, skin-friendly dressings
2.2 In drug delivery, bacterial cellulose can:
- Serve as a local depot for antimicrobials, anti-inflammatory agents, or growth factors
- Be loaded with active ingredients and placed directly on the wound
- Release the drug slowly while keeping the tissue moist and protected
2.3 Market reports show that the bacterial cellulose dressing market is small but fast-growing, with rising use across hundreds of hospitals and companies worldwide. This growth is driven by demand for biodegradable, high-performance wound care materials.
3. Cellulose-Based Hydrogels
3.1 Cellulose-based hydrogels are three-dimensional networks that can hold a large amount of water. Because of this, they are very useful for:
- Topical formulations such as gels and patches
- Injectable depots that form a gel in situ
- Implantable systems for long-term release
3.2 In many cases, formulators combine cellulose with other polymers to create hybrid hydrogels. For example:
- HPMC-based hydrogels reinforced with CNCs for greater strength
- CNF-reinforced hydrogels that resist tearing but still allow diffusion
3.3 The cellulose hydrogels can:
- Protect fragile molecules
- Provide controlled diffusion and release
- Support regenerative medicine, such as cell or growth-factor delivery to injured tissues
As a result, they represent a major class of cellulose-based delivery systems beyond traditional solid dosage forms.
4. Cellulose Derivatives in Oral Controlled Release
The oral route is still the most common way to deliver drugs. Here, cellulose derivatives are workhorse excipients in many controlled-release strategies:
4.1 Matrix tablets
- HPMC swells to form a gel layer
- The drug diffuses out slowly through the hydrated matrix
4.2 Coated tablets and capsules
- EC and cellulose esters can create sustained-release or enteric coatings
- These coatings protect drugs from stomach acid and release them further down the GI tract
4.3 Floating or mucoadhesive systems
- Swelling cellulose derivatives can keep dosage forms in the stomach for longer
- This creates gastric-retentive delivery for drugs that act better in the upper GI tract
The growing cellulose derivatives market reflects how heavily pharma depends on these materials to design cellulose-based delivery strategies for oral dosage forms.
To turn these cellulose-based strategies into fully customized delivery solutions, visit our Targeted Delivery Module Systems and discover how Creative Biolabs can support your formulation design.
Formulation & Design Considerations for Cellulose-Based Systems
Although cellulose is versatile, getting the formulation right still requires careful design. Key levers include:
Degree of substitution (DS)
- Controls hydrophilicity and swelling
- Affects how fast water enters and how fast drugs leave the matrix
Crystallinity
- Higher crystallinity often slows down water uptake and release
- Nanocellulose can offer high crystallinity with tuned surface chemistry
Porosity and particle size
- Larger pores or smaller particles can speed up the release
- Dense structures can delay it and smooth the curve
Crosslinking density in hydrogels
- More crosslinks often mean stronger networks and slower diffusion
Surface modification
Carboxylation, cationization, or ligand conjugation can change:
- Drug loading capacity
- Interaction with APIs (electrostatic forces, hydrogen bonding)
- Targeting certain cells or tissues
By adjusting these parameters, formulators can create cellulose-based delivery strategies that match a desired release profile, from fast onset to ultra-slow long-acting release.
Safety, Regulatory, and Scalability Considerations
From a safety point of view, many cellulose derivatives are already well understood:
- MCC, HPMC, and EC are widely used as excipients
- They are often considered safe within approved limits and appear in many commercial products
For nanocellulose and more novel cellulose structures, the picture is still evolving:
- Early data suggest good biocompatibility
- However, more systematic cytotoxicity and long-term in vivo studies are needed
- Regulatory paths may be longer for completely new cellulose-based devices or excipients
On the manufacturing side, key challenges include:
- Maintaining consistent viscosity, particle size, and purity
- Controlling endotoxin levels and impurities for medical use
- Scaling up nanocellulose and bacterial cellulose with GMP-grade quality
Despite these challenges, the strong safety record of many cellulose derivatives makes them an attractive base for scalable cellulose-based delivery systems.
How Creative Biolabs Supports Custom Cellulose-Based Delivery Projects
Because every project has different needs, custom design is often more powerful than off-the-shelf materials. At Creative Biolabs, cellulose-based platforms are integrated into our broader targeted delivery solutions.
We can support you by:
-
Selecting the right cellulose type for your application:
- HPMC, EC, MCC for oral controlled release
- CNCs, CNFs, and cellulose nanoparticles for nanocarrier systems
- Bacterial cellulose films and cellulose-reinforced hydrogels for local delivery
-
Engineering surface modifications and ligands
- Carboxylated or cationic nanocellulose
- Targeting ligands such as antibodies, peptides, or sugars
- Integration with module-based delivery systems
-
Performing detailed characterization
- Particle size, zeta potential, and morphology
- Drug loading and encapsulation efficiency
- In vitro release profiles and stability
-
Supporting in vitro and in vivo evaluation
- Matching the route of administration and indication
- Building a data package that supports regulatory discussions
For Research Use Only. Not for Clinical Use.
Related Services You May Be Interested in
FAQs
What is cellulose used for in drug delivery?
Cellulose and its derivatives are used as excipients and carriers. They help shape tablets, protect the drug, control how fast it is released, and can even act as active nanocarriers in more advanced systems.
Why choose cellulose-based materials instead of synthetic polymers?
Cellulose comes from renewable sources and is often biocompatible, biodegradable, and low in toxicity. It can offer film-forming and binding properties that equal or even beat many petroleum-based polymers, while also fitting "green" and plant-based trends.
What are nanocellulose drug delivery systems?
Nanocellulose systems use cellulose nanocrystals or nanofibers as tiny carriers with a large surface area. They can be chemically modified to hold drugs and then release them in a controlled way, for example, in oral, topical, or transdermal delivery.
How is bacterial cellulose used in wound dressings and local delivery?
Bacterial cellulose can form soft, hydrated membranes that follow the skin surface, keep the wound moist, and support healing. When loaded with antimicrobials or growth factors, these dressings also work as local drug delivery systems.
Are cellulose-based delivery systems safe and biocompatible?
For well-established grades like MCC, HPMC, and EC, the safety profile is strong, with many approved products on the market. For newer nanocellulose systems, early data are promising, but more detailed long-term studies are still needed.
What are the main challenges of cellulose-based drug delivery?
The biggest challenges are reproducible nanostructure and surface chemistry at scale, consistent drug loading, stable long-term release, and strict regulatory demands for new excipients and devices. Costs for very specialized medical-grade materials can also be high.
Which industries are driving demand for cellulose-based delivery materials?
Pharmaceuticals are a major driver, especially in controlled-release tablets and capsules. Construction, packaging, and broader healthcare applications, such as wound dressings and medical packaging, also contribute to rising demand.
What are the main challenges of cellulose-based drug delivery?
A partner like Creative Biolabs can help you choose the right cellulose format, design surface modifications, and integrate targeting ligands. We also match your API and indication with suitable targeted delivery modules, from in vitro screening to in vivo studies and manufacturability planning.
Conclusion
Cellulose-based delivery systems sit at a powerful intersection of safety, sustainability, and performance. From classic oral tablets based on MCC and HPMC, to cutting-edge nanocellulose carriers, bacterial cellulose wound dressings, and cellulose-reinforced hydrogels, they offer a flexible toolkit for modern drug developers. However, turning cellulose from a simple excipient into a high-value delivery platform requires the right combination of material choice, surface engineering, release design, and biological testing. This is where expert support becomes essential.
If you are exploring cellulose-based delivery strategies for small molecules, biologics, or nucleic acids, our team at Creative Biolabs is ready to help. By combining cellulose platforms with our targeted delivery modules and broad experience in formulation and mechanism-focused studies, we can work with you to design systems that are not only innovative but also scalable and aligned with your regulatory path.
References
- Ciolacu, D. E., Nicu, R. & Ciolacu, F. "Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems." Materials 13, 5270 (2020). https://www.mdpi.com/1996-1944/13/22/5270.
- Liang, S. "Advances in drug delivery applications of modified bacterial cellulose-based materials." Front. Bioeng. Biotechnol. 11, 1252706 (2023). https://www.frontiersin.org/articles/10.3389/fbioe.2023.1252706/full.
- Kupnik, K., Primožič, M., Kokol, V. & Leitgeb, M. "Nanocellulose in Drug Delivery and Antimicrobially Active Materials." Polymers 12, 2825 (2020). https://www.mdpi.com/2073-4360/12/12/2825.Distributed under Open Access license CC BY 4.0, without modification.



