Lipid-Conjugated Antisense Oligonucleotide (ASO) Development Service
Creative Biolabs is a leader in the field of gene medicine, pioneering advanced oligonucleotide delivery strategies. This comprehensive scientific article, authored by our experienced team of PhDs, delves into the powerful capabilities and rigorous development process of our lipid-conjugated antisense oligonucleotide (ASO) development service, providing researchers and pharmaceutical partners with a state-of-the-art platform to fully realize the therapeutic potential of nucleic acid drugs.
ASO Conjugation Introduction
Antisense oligonucleotides (ASOs) represent a pivotal class of therapeutics in modern pharmacology. These short, synthetic nucleic acid sequences are designed to bind specifically to target messenger RNA (mRNA) or pre-mRNA, inducing gene silencing through mechanisms such as RNase H-mediated degradation, steric hindrance, or splice modulation. Despite their exquisite sequence specificity, the inherent physiochemical properties of ASOs—namely their anionic charge, large size, and susceptibility to nuclease degradation—pose significant barriers to efficient cellular uptake and targeted delivery in vivo.
Lipid-Conjugated Antisense Oligonucleotides
Lipid-coupled antisense oligonucleotides (L-ASOs) are chimeric molecules in which one or more lipid moieties are covalently linked to an oligonucleotide scaffold. This coupling has several synergistic functions:
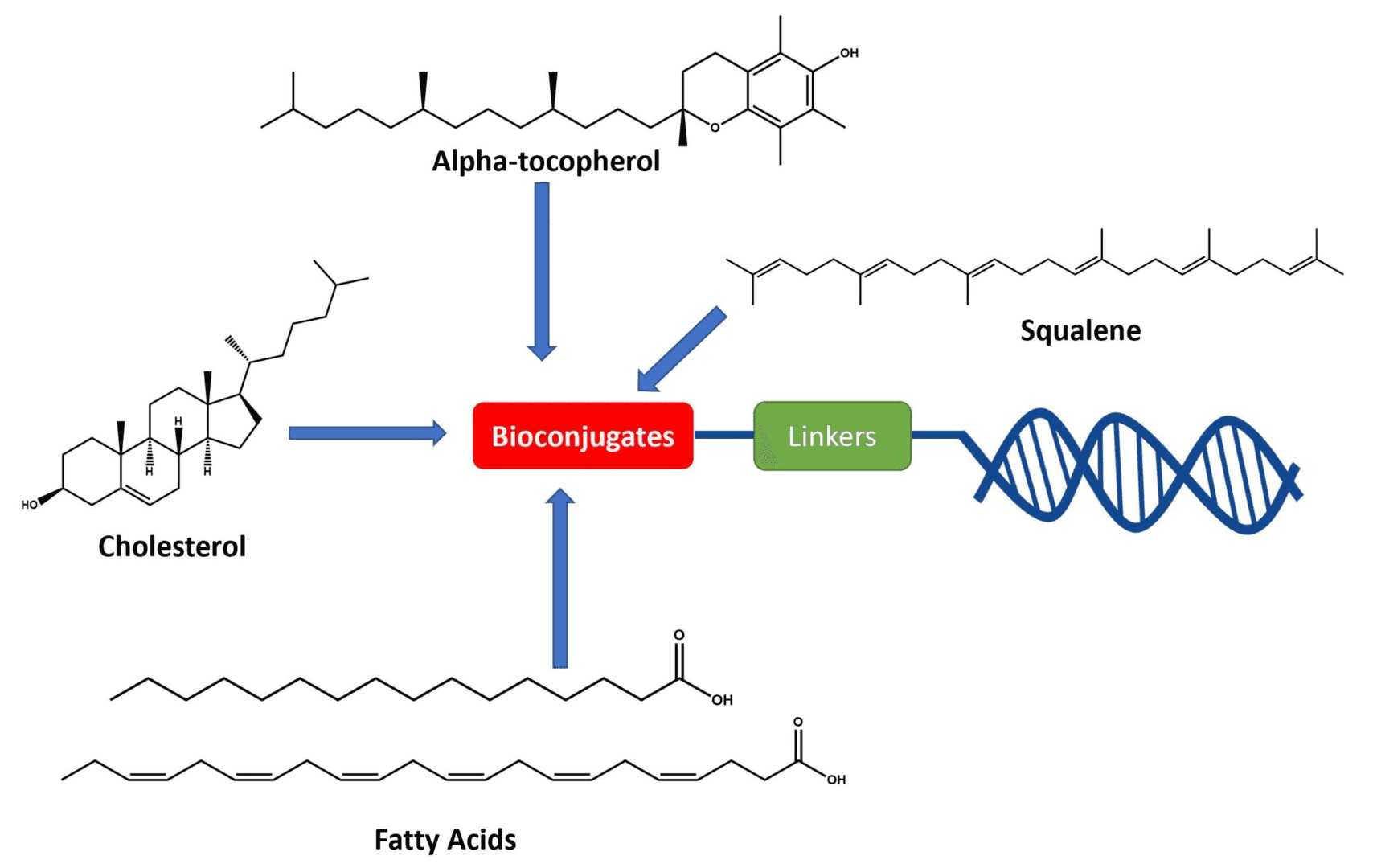 Figure 1 Structure of lipid-coupled oligonucleotides1,3
Figure 1 Structure of lipid-coupled oligonucleotides1,3
Enhanced plasma pharmacokinetics
The hydrophobic lipid linkage increases the binding affinity of ASOs to serum albumin and other circulating proteins.
Promoted cellular uptake
They can promote adsorption-mediated endocytosis or interact with specific lipid transporters, significantly increasing the total amount of ASO internalized compared to uncoupled ASOs.
Improved tissue tropism
Traditional ASOs primarily accumulate in the liver and kidneys, while certain lipid conjugates can redirect ASOs to other tissues. This opens new avenues for treating diseases affecting these organs.
Endosomal escape potential
Some lipids are thought to facilitate the release of antisense oligonucleotides (ASOs) into the cytosol for binding to target RNAs through fusion or a "proton sponge" effect that disrupts the endosome membrane.
Applications of Lipid- Antisense Oligonucleotides Conjugates
Real-time Imaging of Living Cells
L-ASO binds nucleic acid aptamers for specific target recognition. Imaging probes that are developed in combination with LON can be used for targeted cancer bioimaging.
Targeted Drug Delivery
L-ASO has the advantages of low cytotoxicity, controllable size, and available drug-loading sites. When combined with specific targeting ligands, LON can be developed as an efficient delivery vehicle for targeted cancer drugs.
Gene Therapy
L-ASO is simple to synthesize and modify, of high stability, and good safety profile. The lipid moiety can efficiently facilitate the delivery of various nucleic acid drugs and thus functions.
Immunotherapy
The use of L-ASO to modify effector cells can enhance their ability to recognize and attack cancer cells. The immune cells gained recognition ability after modification with aptamers through lipid-tail linkage.
Lipid-Antisense Oligonucleotides Conjugates Synthesis and Purification
Synthetic Strategies
- Pre-synthetic Approach: Lipid modification is carried out in three ways: the hydrophobic moiety is functionalized on the solid phase support at the 3' end of ASO; the hydrophobic phosphoramide bond is coupled at the 5' end. Nucleotides/nonnucleic acids modified by hydrophobic groups are inserted between consecutive oligonucleotides.
- Post-synthetic Approach: The modified lipid fraction is inserted after complete synthesis and purification of the ASO. Since the post-synthetic approach is mostly performed in the liquid phase, it is not easy to purify compared to the pre-synthetic approach in solid-phase synthesis.
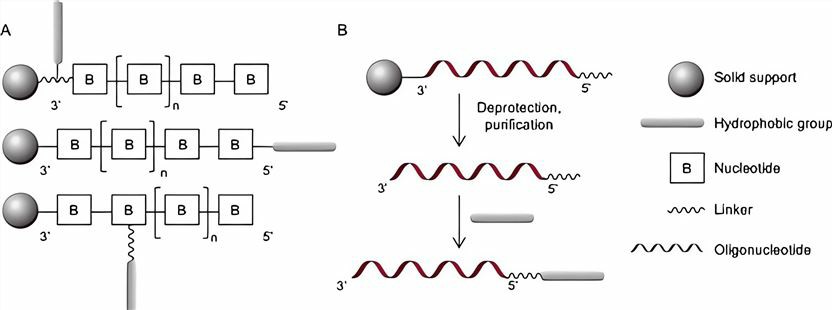 Figure 2 Pre-synthetic approach and post-synthetic approach.2,3
Figure 2 Pre-synthetic approach and post-synthetic approach.2,3
Purification and Analysis
- Tangential Flow Filtration (TFF): An efficient initial step for desalting and concentrating the sample, removing small molecule impurities.
- RP-HPLC: This is the primary method for purifying lipid-coupled ASO, effectively separating the more hydrophobic conjugate from the unmodified oligonucleotide based on retention time.
- IEX-HPLC: It provides an orthogonal purification method that separates different substances based on their charge, which is particularly important for identifying and removing ineffective sequences and ensuring product homogeneity.
Conjugation Technology for Lipid-Antisense Oligonucleotides Conjugates
We possess advanced technological capabilities, thanks to our state-of-the-art instrumentation and methods. We utilize automated synthesizers to ensure the reproducibility and large-scale production of our reactions. At the heart of our conjugation technology lies our comprehensive toolkit of linker chemistry and bioorthogonal reactions.
Linker Chemistry: The chemical spacer connecting antisense oligonucleotides (ASOs) and lipids is not a simple linker, but a crucial functional element. We design and utilize a variety of linkers:
- Cleavable Linkers: These linkers remain stable in the circulatory system but are degraded in the cellular environment (e.g., by intracellular reducing agents such as specific enzymes in the endosome-lysosome pathway), releasing the active ASO.
- Uncleavable Linkers: These stable linkers (e.g., polyethylene glycol, PEG, or simple alkyl chains) maintain the covalent connection. The bioactivity of the conjugate depends on whether the ASO can hybridize with its target while being linked to the lipid.
- EG Spacer: Introducing a PEG unit between the ASO and lipid can improve water solubility, reduce aggregation, and modulate the distance between functional units, thereby affecting bioactivity.
Our scientists work closely with our clients to select the optimal coupling site (5' end, 3' end, or internal), linker chemistry, and reaction conditions to achieve the desired therapeutic effect.
Our Services
Fully functional L-ASO is composed of three different segments, (1) a well-designed and modified ASO, (2) the linker, and (3) lipid derivatives. Synthetic L-ASO is produced by either a pre-synthetic or post-synthetic approach, in which lipid-like moieties can be introduced in different ways. Creative Biolabs offers an end-to-end, collaborative service for the development of your lipid-conjugated ASO therapeutics. Our integrated platform covers every stage from design to delivery of a characterized candidate.
- Sequence Design & Optimization
- Custom Lipid Selection & Sourcing
- Chemical Synthesis & Conjugation
- Comprehensive Purification
- Comprehensive Impurity Profiling
- In Vitro and In Vivo Screening
Types of Lipids for Antisense Oligonucleotides Conjugation
The selection of the lipid moiety is a key factor determining the overall performance of the conjugate. Our service platform has extensive experience in lipid conjugation, as each lipid possesses unique properties and applications. The table below summarizes the main categories:
| Lipid Category | Representative Examples | Key Properties & Mechanisms |
|---|---|---|
| Long-Chain Fatty Acids | Palmitic Acid, Stearic Acid |
|
| Steroid-Based Lipids | Cholesterol |
|
| Vitamin-Derived Lipids | α-Tocopherol (Vitamin E) |
|
| Phospholipid Mimetics | Polar head-group analogs (e.g., choline, ethanolamine derivatives) |
|
| Novel Synthetic Lipids | Proprietary structures |
|
Analytical Characterization & QC
Rigorous analytical characterization is essential. For each batch, we provide a comprehensive certificate of analysis, including:
- Purity analysis: IP-RP-HPLC and/or IEX-HPLC (purity standards >95%).
- Identification: Electrospray ionization (ESI) or matrix-assisted laser desorption/ionization (MALDI) mass spectrometry.
- Structural validation: Sequence and modification confirmation using tandem mass spectrometry (MS/MS).
- Concentration and yield quantification: UV-Vis spectrophotometry.
- Sterility and endotoxin detection: Applicable to conjugates intended for in vivo studies.
Advantages of Lipid- Antisense Oligonucleotides Conjugates
The following advantages of L-ASO facilitate its application in the biomedical field:
High Bioavailability
Lipid-cell membrane interactions promote efficient intracellular transport and endosome release of L-ASO, thereby improving its solubility, drug loading capacity, and transmembrane mobility.
Ease of Synthesis
L-ASO can be synthesized more conveniently, efficiently, and at a lower cost using conventional chemical methods. Furthermore, various chemical modifications are easily introduced.
Enhanced Stability
Lipid conjugation enhances the hydrophobicity of ASO and increases its molecular weight, thereby protecting ASO from nuclease degradation, prolonging the drug's half-life and efficacy.
Good Safety Profile
Lipid-conjugated ASO reduces the risk of immunogenicity and maintains tolerability even with high-dose administration in vivo.
Frequently Asked Questions
Q: What is your minimum production scale?
A: We can start projects in the milligram range (typically 10 mg to 50 mg) for early in vitro screening and proof-of-concept studies, ensuring no material is wasted.
Q: How do you ensure the stability of the lipid-ASO bond?
A: For most applications, we use a stable, non-hydrolyzable linker at the 5' end. For designs requiring in vivo release, we provide accelerated and real-time stability data of the cleavable linker itself to confirm its integrity under storage conditions and its responsiveness to specific release stimuli (e.g., low pH, enzyme activity).
Q: Besides lipid conjugation, do you offer other modification services?
A: Absolutely. Our core expertise includes a full range of backbone and glycosyl modifications (e.g., 2'-OMe, 2'-MOE, LNA), which are often combined with lipid conjugation to enhance nuclease resistance and binding affinity. Lipid conjugation is always performed on fully modified ASO.
Q: What is the typical turnaround time for custom conjugation?
A: Turnover time depends on the size and complexity of the lipids and linkers required.
Connect with Us Anytime!
The development of lipid-conjugated antisense oligonucleotides is not a simple synthetic process, but a precise pharmacological practice requiring specialized knowledge and skills to design molecules that can successfully overcome physiological barriers and reach gene targets. Creative Biolabs is ready to be your strategic partner in this field. With advanced chemical technologies, rigorous quality control, and comprehensive regulatory compliance, we ensure that your novel antisense oligonucleotide (ASO) concepts can be translated into highly effective, pure, and viable therapeutic candidates, thereby accelerating the development of next-generation gene therapy. Contact us today for a quotation or any question. Our customer service representatives are available 24 hours a day!
References
- Tran, Phuc, et al. "Delivery of oligonucleotides: efficiency with lipid conjugation and clinical outcome." Pharmaceutics 14.2 (2022): 342. 10.3390/pharmaceutics14020342 Distributed under Open Access license CC BY 4.0 without modification.
- Li, Xiaowei, et al. "Lipid–oligonucleotide conjugates for bioapplications." National Science Review 7.12 (2020): 1933-1953. 10.1093/nsr/nwaa161 Distributed under Open Access license CC BY 4.0, The image was modified by extracting and using only Part A,B of the original image.
