The properties of the lipid formulations can vary according to the composition (cation, anion, neutral lipid species), but the same preparation method can be used for all lipid vesicles regardless of the composition. The general elements of the procedure include preparing lipids for hydration, hydrating and sizing with stirring until the vesicles are evenly distributed.
The rigidity of bilayer membrane is an important parameter to be considered in the study of liposome formation. The hydrated single component phospholipid bilayer can be in liquid crystal ("fluid") state or gel state. With the increase of temperature, the gel bilayer membrane melts and transforms into liquid. This occurs at a temperature called the transition temperature (Tc). The Tc of bilayers depends on:
1. Acyl chain length.
2. Degree of saturation.
3. Polar head group.
The raw materials for the formation of liposomes depend on the intended use of liposomes. The preparation process of liposomes could be achieved by using three different techniques, such as the mechanical methods, the solvent dispersion methods, and methods based on fusion or size transformation of the prepared vesicle.
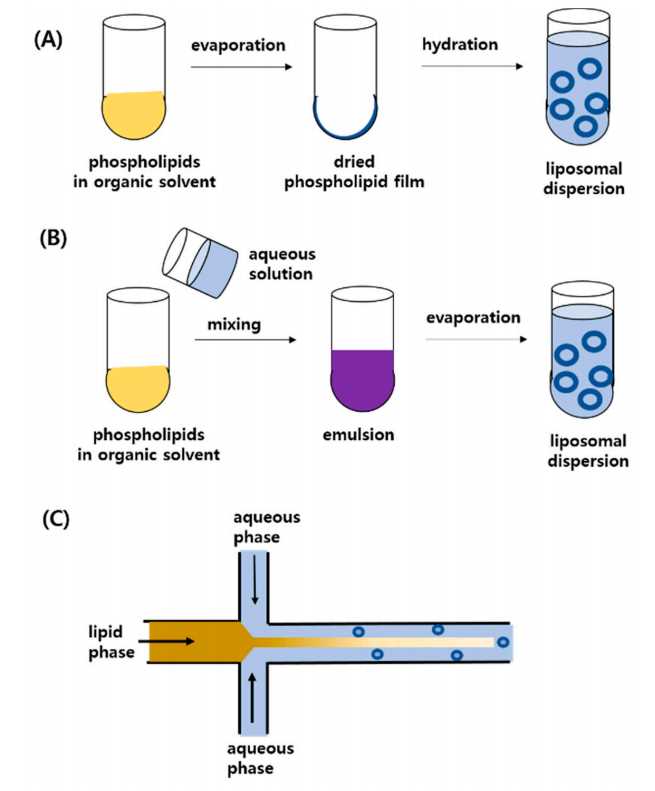 Fig.1 Representative techniques for the preparation of liposomes.1,2
Fig.1 Representative techniques for the preparation of liposomes.1,2
The method is to hydrate the lipid film in organic solvent, and then remove the organic solvent in vacuum to prepare liposomes. After the solvent was completely removed, the solid lipid mixture was hydrated by aqueous buffer. Liposomes were formed by spontaneous swelling and hydration of lipids. The main disadvantages of this method are low encapsulation rate, difficult to expand and uneven size distribution.
SCF has been introduced into the preparation of liposomes to overcome the problems of traditional methods, such as the need for a large number of toxic organic solvents and limited laboratory scale production. Supercritical carbon dioxide is the most commonly used supercritical fluid for the preparation of liposomes. It has the advantages of non-toxic, non combustible, recyclable, easy to remove from the solvent, moderate operating temperature and avoiding the degradation of the product in inert atmosphere.
Some researchers have used microfluidic technology, namely microemulsion technology, to prepare liposomes. Based on the preparation of liposomes by thin-layer hydration method, the homogenized particles were obtained by ultrasonic treatment and microfluidic control with bath-type sonicator. The process of microfluidization control has good repeatability, and the obtained liposomes have good encapsulation property in aqueous phase.
In this method, a lipid mixture is added to a round bottom flask and organic solvents (ether and isopropyl ether) are removed under reduced pressure by a rotary evaporator. Liposomes are formed when residual solvents are removed by continuous rotary evaporation under reduced pressure. This method can encapsulate macromolecules efficiently and achieve high encapsulation efficiency (up to 65%) in low ionic strength media (e.g. 0.01 M NaCl). The main disadvantage of this method is that the solvent may remain in the formulation and it is difficult to scale up.
In this method, the mixture of lipid and organic solvents (ether, ethanol, etc.) is slowly injected into a warm aqueous solution. This results in osmotic activity, clear size distribution of unilamellar vesicles and high volume capture efficiency (about ten times higher than that of sonicated and hand shake preparations).
This method is based on size transformation, which includes the subsequent sonication treatment of MLV prepared by thin-film hydration method, and the use of acoustic energy in inert atmosphere (including nitrogen or argon). The sonication method can make the vesicles disperse evenly by using the bath or probe type ultrasonic instrument, and has greater tissue penetration potential. However, probe tip sonicator will transfer high energy to lipid suspension, so this method is not suitable for the encapsulation of thermolabile substances.
In this method, the liposome formed by the film method rotates with the solute to be encapsulated until the whole membrane is suspended. Then the liposomes are frozen in dry ice ethanol (-80˚C) or in liquid nitrogen, thawed, and rotated again. The freeze-thaw cycle is repeated. This method is widely used in protein encapsulation.
MLVs prepared by thin-film hydration method are repeatedly passed through filters polycarbonate membranes reducing the liposome size in high-pressure extrusion method. The liposomes are prepared using thin-film hydration method subsequently using an extruder for ten cycles to obtain extruded liposomes with uniform diameters.
This method is used to prepare LUV liposomes from acidic phospholipids. The addition of calcium to SUV liposomes induces fusion and leads to the formation of multilamellar vesicles. The addition of EDTA to the formulation resulted in the formation of LUV liposomes. The advantage of calcium-induced fusion method is that it can encapsulate large molecules, but the disadvantage is that LUV liposomes can only be obtained from acidic phospholipids.
We have strong and extensive expertise and years of experience in the field of drug delivery and liposome development services. For more details about our services, please directly contact us.
References
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry