Autoimmunity on Mute-siRNA's Assault on Inflammatory Diseases
Introduction of siRNA in Autoimmunity Regulation
Scientists discovered RNA interference (RNAi) in Caenorhabditis Elegans during 1998. Small mRNA research led scientists to identify small-interfering RNAs (siRNAs) which they further investigated to confirm their direct effect on translation. The size of siRNA molecules extends from 15 to 30 base pairs in length. siRNAs silence target genes through mRNA translation inhibition and mRNA degradation acceleration. Significant breakthroughs in siRNA therapeutic development occurred because pharmaceutical companies invested substantial resources which helped achieve clinical translation success. Patisiran became the first liposome complex approved for siRNA binding to treat hereditary transthyretin-mediated amyloidosis in 2018. The swift advancement of siRNA benefits from the progress in lipid nanoparticles (LNPs) technology combined with related nucleic acid modification methods. Some progress has been made by researchers who use siRNAs to treat autoimmune diseases.
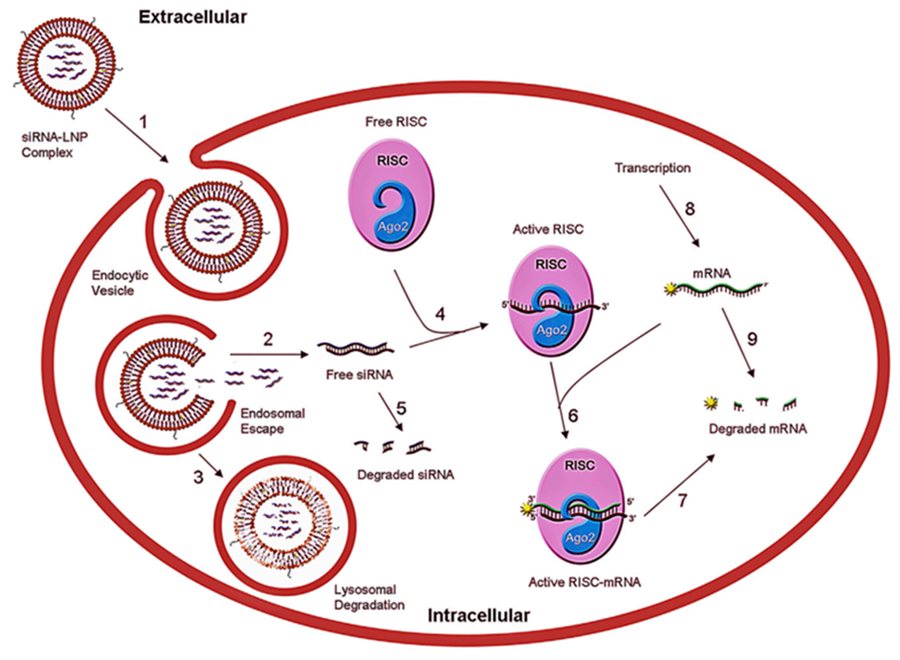 Fig. 1 Schematic representation of mechanism of LNP-mediated siRNA delivery into cells resulting in gene silencing1,7.
Fig. 1 Schematic representation of mechanism of LNP-mediated siRNA delivery into cells resulting in gene silencing1,7.
Introduction of Autoimmunity
- The Rising Tide of Autoimmune Diseases
The immune system attacks healthy body parts by mistake during autoimmune diseases which causes persistent inflammation and tissue damage. Rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), and inflammatory bowel disease (IBD) rank among the most common autoimmune diseases that strike millions throughout the world. The rising number of autoimmune disease cases creates extensive stress for healthcare systems. Rheumatoid arthritis impacts nearly 1% of people worldwide yet systemic lupus erythematosus and multiple sclerosis show growing incidence rates across numerous areas.
- Current Treatments
Medical treatment options for autoimmune diseases focus on the use of immunosuppressive medications together with biologic therapies. Autoimmune disease treatments reduce symptoms and inflammation yet their effectiveness is restricted by significant limitations. The broad effect of immunosuppressants such as corticosteroids and DMARDs results in higher infection risks alongside other negative side effects. Monoclonal antibodies along with receptor fusion proteins direct their effects at specific inflammatory pathways but tend to result in negative side effects while failing to sustain remission among various patients. The treatment of RA with biologics such as monoclonal antibodies proves effective but their extended use creates a heightened risk of infections and additional complications.
- siRNA as a Precision Weapon
siRNA provides a unique treatment option for autoimmune diseases through its ability to silence particular genes that drive the development of these conditions. Unlike conventional therapies which broadly suppress immune functions siRNA precisely targets malfunctioning immune signals to reduce inflammation and tissue damage while maintaining overall immune functionality. Preclinical RA models demonstrate that siRNA targeting TNF-α shows potential to decrease both inflammation and joint damage. NF-κB targeted by siRNA has been effective in controlling inflammation across different autoimmune models since NF-κB plays a crucial role in immune response regulation. By focusing treatment on specific targets this approach delivers better results with reduced side effects while overcoming current therapy limitations and providing new hope for autoimmune disease management.
siRNA's Battle Plan: Strategic Gene Silencing
- Target Pro-inflammatory cytokines
TNF-α acts as a leading pro-inflammatory cytokine that stimulates RA progression. TNF-α functions as an essential regulator of cytokine production and continuous chronic inflammation development. Experimental research reveals that siRNA targeting TNF-α mRNA reduces inflammation while protecting joints from damage in animal models of RA. A study created a nanocomplex consisting of polymerized siRNA directed at TNF-α combined with thiolated glycol chitosan (tGC) polymers which formed psi-tGC-NPs. The delivery system proved fast cellular absorption and outstanding TNF-α gene silencing effectiveness during in vitro testing. CIA mice demonstrated that psi-tGC-NPs gathered at arthritic joints where they significantly reduced inflammation and prevented bone erosion. This method presents a precise approach to lower TNF-α levels which helps reduce rheumatoid arthritis symptoms.
- Target Immune cell activation-Blocking CD40/CD40L in lupus
The CD40/CD40L signaling pathway controls immune cell activation and expansion which proves vital in autoimmune disease processes with systemic lupus erythematosus (SLE) serving as a key example. Inhibiting the CD40/CD40L pathway reduces the activation of both T cells and B cells leading to reduced autoimmune responses. Researchers can develop siRNA to bind CD40 or CD40L mRNA and subsequently lower these proteins' expression while reducing immune cell activation. Research demonstrates that the CD40L-Mac-1 interaction blockade via siRNA treatment leads to diminished inflammation in acute peritonitis models while maintaining normal immune functions. Targeted modulation provides precise immune response control while preserving overall immune system function to minimize side effects.
- Target Autoantibody production
B-cell activating factor (BAFF) and B-lymphocyte stimulator (BLyS) cytokines support B-cell survival and autoantibody production which act as fundamental mechanisms in autoimmune diseases including SLE. Application of siRNA to BAFF and BlyS results in decreased B-cell survival and activation which then leads to lower autoantibody production. Studies show that using siRNA to target BAFF decreases B-cell survival as well as autoantibody production in preclinical SLE models. This targeted strategy delivers precise intervention while ensuring effective treatment to reduce autoantibody-induced harm in autoimmune disorders.
- Case study: siRNA vs. psoriasis (IL-17/IL-23 axis)
Psoriasis persists as an inflammatory skin disease due to increased keratinocyte growth along with immune cell invasion. IL-17 and IL-23 cytokines are essential for psoriasis progression because they drive inflammation and keratinocyte proliferation. Therapeutic use of siRNA against IL-17 and IL-23 mRNA shows great promise in inflammation reduction and better psoriasis treatment results. Research shows that siRNA molecules targeting IL-17 produce diminished cytokine expression in psoriatic skin models and simultaneously decrease inflammation and enhance skin appearance. Researchers propose this targeted method as an effective treatment for psoriasis which selectively inhibits major inflammatory mediators while preserving other cellular activities.
Delivery Tactics: Crossing the Fortress Walls
Challenge: siRNA degradation, reaching immune cells
Targeting immune cells like macrophages and T cells presents major obstacles for siRNA delivery and function. The main obstacles for siRNA delivery are its quick breakdown by nucleases outside cells and its requirement to cross cell membranes effectively. The mononuclear phagocytic system (MPS) quickly removes siRNA from the bloodstream which siRNA must avoid to remain effective. The current obstacles prevent siRNA from accessing target cells to perform its therapeutic function.
Solutions
- LNPs: Liver-Focused
LNPs represent a top-tier delivery method for siRNA. The protective capability of LNPs against siRNA degradation helps deliver therapeutic siRNA to its target cells. Alnylam Pharmaceuticals has created LNPs that deliver siRNA to the transthyretin (TTR) gene in patients suffering from hereditary transthyretin amyloidosis (hATTR). The application of this technology yielded successful results with patisiran which patients receive through intravenous administration to achieve substantial TTR protein reduction. LNPs can be modified to deliver siRNA to particular cell types through the addition of targeting ligands which improve their delivery capabilities to immune cells.
- Peptide-Conjugated siRNA: Homing to Inflamed Tissues
The use of peptide-conjugated siRNA represents a novel method for achieving targeted delivery. Researchers used siRNA attached to an RVG-derived peptide from the rabies virus glycoprotein to target macrophages and microglial cells within neuroinflammation models in their study. The delivery system demonstrated ability to suppress TNF-α expression among pro-inflammatory cytokines within these cells during in vitro and in vivo experiments. Researchers can create specialized peptides which target inflamed areas including RA joints to direct siRNA to inflammation sites where it diminishes cytokine production.
- Exosome Delivery: Natural Vesicles for Immune Cell Targeting
Small vesicles secreted by cells known as exosomes show great potential as delivery systems for siRNA. These vesicles demonstrate natural targeting abilities for immune cells while delivering siRNA without initiating immune reactions. Studies demonstrate that exosomes containing siRNA directed against certain immune cell markers effectively silence genes within macrophages and T cells. The method utilizes exosomes' inherent capacity to traverse biological barriers so they can deliver cargo to defined cell types which establishes it as a potent technique for immune cell targeting.
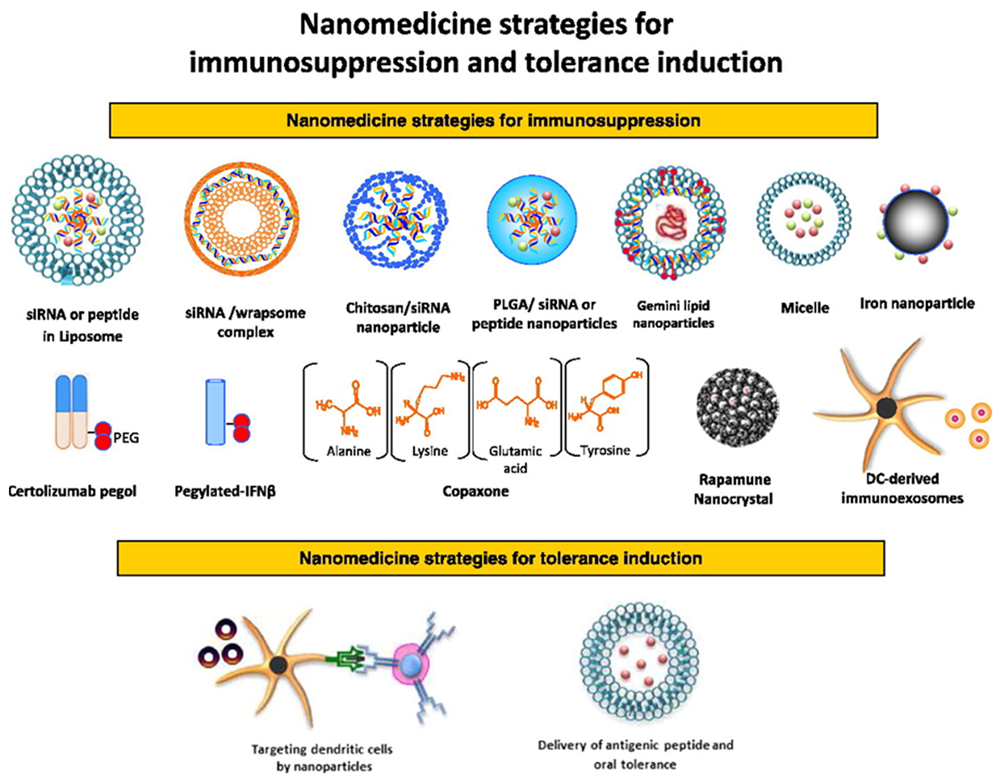 Fig.2 The use of nanomedicine in treating autoimmune diseases: advancing from immunosuppression to promoting immune tolerance2,7.
Fig.2 The use of nanomedicine in treating autoimmune diseases: advancing from immunosuppression to promoting immune tolerance2,7.
siRNA in preclinical study of Autoimmunity
- siRNA in Sjögren's syndrome
Sjögren's syndrome (SjS) represents an autoimmune disease which presents as dry mouth and dry eye symptoms. Through the infiltration of self-reactive lymphocytes into the lacrimal and salivary glands self-destructive processes occur which lead to acinar cell apoptosis in those glands during SjS. Researchers used carbachol as a muscarinic receptor-specific ligand and secretagogue in an in vitro study before conjugating it with an siRNA that silences caspase 3 expression. The study utilized a human salivary gland (HSG) cell line to test the effectiveness of the secretagogue-siRNA conjugate. The secretagogue-siRNA conjugate treatment led to decreased caspase-3 gene/protein expression levels and blocked cytokine-triggered apoptosis in HSG cell line epithelial cells which represents a crucial discovery for sustaining fluid secretion processes in SjS. Patients with SjS currently depend on immunosuppressive drugs and secretagogues to stimulate secretion for treatment. The in vitro experiments previously described suggest that siRNA-based silencing methods could prevent apoptosis in secretory acinar cells in SjS patients thereby preserving cell viability and secretory functions which would translate into enhanced quality of life.
- siRNA in RA
Rheumatoid Arthritis stands as the predominant auto-inflammatory arthritis affecting joints throughout the general population at a rate of nearly one percent. The development of RA emerges from complex interactions among environmental and genetic factors together with epigenetic changes that cause immune tolerance breakdown leading to synovial inflammation. Different processes work together to trigger both the breakdown of the extracellular matrix and inflammatory responses. The effectiveness of siRNA-based treatment for RA has been assessed through multiple research studies. Researchers utilized localized electroporation for siRNA delivery into joint tissues in a study conducted with the CIA mouse model. Electroporation of siRNA targeting TNF-α was effective in preventing joint inflammation in mice according to the study. A separate investigation used DNA complexation and a cationic liposome system to intravenously administer the TNFα-targeting siRNA in the CIA mouse model. The TNFα-targeting siRNA treatment led to a 50%-70% reduction in TNF-α levels and complete elimination of arthritic symptoms in CIA mice. Following intravenous dosing, TNFα-targeting siRNA became absorbed by multiple tissues including the lungs, brain, spleen, and liver.
- siRNA in autoimmune skin diseases
A preclinical study presented the development of a JAK1-selective siRNA designed for controlling skin autoimmunity. Researchers discovered the lead compound siRNA 3033 via computational screening and validated its ability to suppress JAK1 expression in both human and mouse skin models. In a mouse model of autoimmune skin disease vitiligo researchers tested this siRNA which functions through IFN-γ signaling. The research showed notable therapeutic success through the functional blockade of JAK1-dependent inflammatory pathways. The study demonstrates how siRNA treatment shows promise for managing inflammatory and autoimmune conditions of the skin.
Advantages Over Conventional Therapies
- Precision: No Broad Immunosuppression (Lower Infection Risk)
siRNA therapy represents an accurate treatment method that allows for precise gene silencing while avoiding broad immunosuppression. Through precise targeting of disease-causing gene mRNA siRNA therapy silences these genes to reduce inflammation and immune activation without suppressing the whole immune system similarly to corticosteroids and other immunosuppressive drugs. The specific therapeutic approach minimizes the risk of infections and systemic immunosuppression-related side effects. In animal research siRNA targeting inflammatory cytokines like TNF-α has been shown to reduce arthritis inflammation while preserving the immune system's overall function.
- Durability: Months-Long Effects from a Single Dose
siRNA therapies provide durable therapeutic outcomes that extend over several months from just one treatment dose. The approach represents a major advancement since traditional treatments typically need repeated dosing. Patisiran delivers treatment for hereditary transthyretin amyloidosis using tri-weekly intravenous injections which maintained proven effectiveness throughout an 18-month study period. Researchers observed that a single dose of Cemdisiran (ALN-CC5) produced powerful gene silencing effects that persisted for over a year during preclinical testing. The extended action period of the drug minimizes the need for repeated doses which helps patients stick to their treatment plans.
- Adaptability: Rapid Redesign for New Targets
Researchers can rapidly modify siRNA therapeutic strategies to target new genetic elements or biological routes because of its highly adaptable nature. The ability to modify therapy quickly provides an essential advantage when dealing with new diseases or changing disease conditions. siRNA therapy allows for fast development of treatments to attack stable sections of viral genomes which enables it to effectively fight viral infections. The engineering flexibility of siRNA allows it to target multiple genes at once which enables a comprehensive treatment strategy for complex diseases. SiRNA therapies demonstrate flexibility by evolving in tandem with disease mechanisms to deliver versatile and potent treatment approaches.
Beyond Autoimmunity of siRNA: Expanding the Horizon
- Allergies: Silencing IgE Production
siRNA therapy reveals substantial promise for allergy treatment because it selectively targets and suppresses genes that drive allergic responses. The CD40 molecule stands out as a vital target because it functions as a critical costimulatory molecule in allergic immune responses. Researchers found that when CD40 was silenced with siRNA it led to the creation of immunoregulatory dendritic cells which reduced both nasal allergic symptoms and eosinophil buildup in mice with ovalbumin-induced allergies. The study demonstrated that siRNA treatment successfully blocked the production of OVA-specific IgE and Th2 cytokines IL-4 and IL-5 which showed how siRNA has the capability to alter immune responses and minimize allergic symptoms. One therapeutic strategy focuses on nerve growth factor (NGF) since it affects airway inflammation and hyperresponsiveness (AHR) in asthmatic conditions. In an asthmatic animal model researchers observed significant reductions in both AHR and airway inflammation after suppressing NGF production via siRNA. This therapeutic strategy demonstrates how siRNA can precisely target molecules that participate in allergic reactions to deliver effective allergy treatments.
- Transplant Rejection: Muting Donor-Specific Immune Responses
siRNA demonstrates potential in transplant rejection prevention through immune response regulation against donor organs. A novel technique employs tolerogenic dendritic cells (Tol-DCs) created through the silencing of the circular RNA FSCN1 (circFSCN1). An investigation found that circFSCN1-silenced Tol-DCs treatment stopped alloimmune rejection and extended allograft survival during murine heart transplantation. The therapeutic intervention decreased fibrosis while promoting regulatory T cell formation which demonstrates how siRNA can establish a tolerogenic milieu to avert transplant rejection. The study investigated siRNA application as a tool to target Siglec-E which regulates antigen-presenting cell function and T cell-mediated transplant rejection in mice. Through immune response modulation to donor organs this treatment presents a potential strategy for transplant rejection prevention.
- Long COVID: Targeting Inflammatory Remnants
Long COVID presents through ongoing symptoms and inflammation after a person experiences acute SARS-CoV-2 infection. siRNA possesses the ability to deactivate genes that drive the persistent inflammatory processes linked to Long COVID. The potential of siRNA to control immune responses and decrease inflammation indicates its promise as a treatment option for the persistent symptoms of COVID-19 despite the ongoing development of specific research in this area. siRNA designed to target pro-inflammatory cytokines and immune cell activation pathways shows promise for decreasing chronic inflammation and enhancing patient outcomes in Long COVID cases.
References
- Kalita, Tutu, et al. "siRNA functionalized lipid nanoparticles (LNPs) in management of diseases." Pharmaceutics 14.11 (2022): 2520. https://doi.org/10.3390/pharmaceutics14112520.
- Parvin, Nargish, Sang Woo Joo, and Tapas K. Mandal. "Biodegradable and Stimuli-Responsive Nanomaterials for Targeted Drug Delivery in Autoimmune Diseases." Journal of Functional Biomaterials 16.1 (2025): 24. https://doi.org/10.3390/jfb16010024.
- de Brito e Cunha, Danielle, et al. "Biotechnological evolution of siRNA molecules: from bench tool to the refined drug." Pharmaceuticals 15.5 (2022): 575. https://doi.org/10.3390/ph15050575.
- Song, Yi, Jian Li, and Yuzhang Wu. "Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders." Signal Transduction and Targeted Therapy 9.1 (2024): 263. https://doi.org/10.1038/s41392-024-01952-8.
- Daub, Steffen, et al. "CD40/CD40L and related signaling pathways in cardiovascular health and disease—the pros and cons for cardioprotection." International journal of molecular sciences 21.22 (2020): 8533. https://doi.org/10.3390/ijms21228533.
- Tang, Qi, et al. "Rational design of a JAK1-selective siRNA inhibitor for the modulation of autoimmunity in the skin." Nature Communications 14.1 (2023): 7099. https://doi.org/10.1038/s41467-023-42714-4.
- Distributed under Open Access license CC BY 4.0, without modification.
