siRNA-The Precision Tool for Genetic Surgery
What is RNA interference
Eukaryotic cells use small double-stranded RNA molecules known as small interfering RNA (siRNA) to activate RNA interference (RNAi) pathways that target and destroy equivalent mRNA sequences resulting in gene silencing. Scientists uncovered specific gene silencing by administering double-stranded RNA to Caenorhabditis elegans cells in 1998. They were awarded the 2006 Nobel Prize in Physiology or Medicine for their discovery that Dicer converts dsRNA into siRNA which directs the RNA-induced silencing complex (RISC) to destroy target mRNA.
siRNA function: Next-Generation RNAi Therapeutics
- Self-Delivering siRNA
Contemporary RNAi therapeutic studies focus on self-delivering siRNA molecules capable of crossing tissue barriers without the need for carrier molecules. This advancement resolves major delivery challenges when directing siRNA to precise organs such as the heart and liver. Self-delivering siRNA molecules achieve improved stability and reduced immunogenicity through their design which leads to more precise targeting of target cells. Preclinical studies indicate that this method shows potential for revolutionizing disease treatment through improved targeted delivery methods.
- Conditional siRNA: Smart Triggers for Tissue-Specific Activation
RNAi therapeutics have gained a new cutting-edge advancement through the development of conditional siRNA. Specific conditions such as the presence of a particular tissue type or disease state activate these siRNA molecules. The intelligent activation system activates siRNA only at targeted sites to minimize off-target effects while enhancing therapeutic accuracy. The ability to design conditional siRNA molecules that activate upon detecting particular cellular signals or environmental changes makes them an effective solution for precise gene silencing in intricate biological systems.
- Simultaneous Targeting of Multiple Disease Pathways
Multiplexed siRNA development marks a key improvement in RNA interference treatment because researchers can now target several disease pathways at the same time. Scientists develop siRNA structures to achieve combined gene suppression in disorders that require multiple gene targets. The multiplexing technique can improve treatment outcomes because it targets different disease mechanisms at once to deliver an all-encompassing treatment plan.
siRNA's antiviral ability
- siRNA-vaccine hybrids: Combining gene silencing with immunization
Researchers have developed siRNA-vaccine hybrids which utilize gene silencing techniques together with standard immunization methods to create an innovative treatment strategy. The hybrid technique utilizes siRNA to precisely attack and deactivate genes that control viral replication and immune evasion which improves vaccine performance. Scientists produce siRNA molecules to target essential viral genes involved in entry and replication and enhance the vaccine-induced immune response. The creation of next-generation COVID-19 vaccines demonstrates a major use of siRNA-vaccine hybrid technology. Research has been conducted on siRNA-based targeting techniques to focus on the SARS-CoV-2 spike protein that functions as a crucial component of current mRNA vaccines. The hybrid vaccine merges siRNA with spike protein encoding mRNA to silence viral genes and prompt a strong immune response which may provide superior defense against new variants.
- Viral escape prevention: Using siRNA as an antiviral booster
Viruses develop mutations that enable them to hide from the immune system which creates major challenges in developing effective antiviral treatments and vaccines. siRNA provides an effective antiviral mechanism because it identifies stable regions within viral genomes which do not change through mutations yet remain essential for viral survival. The precise application of this method successfully blocks viral escape while extending the effectiveness of antiviral treatments. siRNA attacks several conserved sections of the SARS-CoV-2 genome like RNA-dependent RNA polymerase (RdRp) and nucleocapsid (N) protein during COVID-19 treatment to minimize viral escape through broad-spectrum antiviral action.
Case study
- siRNA therapeutic for COVID-19
siRNA therapies provide the benefit of being administered as a cocktail allowing multiple target sequences to be delivered at once. The use of multi-targeting siRNA methods shows promise as an effective treatment option for COVID-19 infections. A recent highly promising method led researchers to pinpoint and generate siRNA for three conserved mRNA sequences of COVID-19. Scientists administered siRNA to attack mRNA for RdRp and Helicase as well as the 5′ untranslated region either in isolation or through simultaneous delivery. Scientists inoculated COVID-19 virus into K18-hACE2 transgenic mice that expressed the human ACE2 receptor. Researchers administered the siRNA within HFDM lipid nanoparticles through retro-orbital injection at doses of 1 mg/kg in 100 ml of solution to mice two days after viral inoculation. The siRNA treatment demonstrated its ability to provide survival benefits through the inhibition of COVID-19 infection.
- siRNA therapeutic for HIV
2005 marked the reporting of the initial siRNA therapeutic developed to epigenetically silence HIV. The siRNA therapeutic known as siPromA targets the tandem NF-κB binding motifs of the 5'LTR viral promoter to achieve powerful viral suppression up to 1000-fold for more than 30 days without causing in vitro off-target effects. The therapeutic effects were linked to repressive epigenetic patterns that lead to gene silencing through methylation of H3K9 and H3K27. Research using lentiviral vectors to maintain constant siPromA expression showed that HIV replication remained suppressed for more than a year and cells expressing siPromA resisted reactivation stimuli. The research team identified a new si143 molecule which targets the 5'LTR located before siPromA and they showed that using multiple therapies together produces silencing effects similar to single therapy treatments. Researchers used mouse in vivo models to test the siRNA therapeutic approach by transplanting mice with human blood mononuclear cells (PBMCs) and CD34+ hematopoietic stem cells (HSCs) modified to express siPromA stably. Gene-modified mice that expressed siPromA showed lower levels of intracellular HIV genomic RNA and enhanced protection of CD4+ T cells from virus-induced death when compared to control groups.
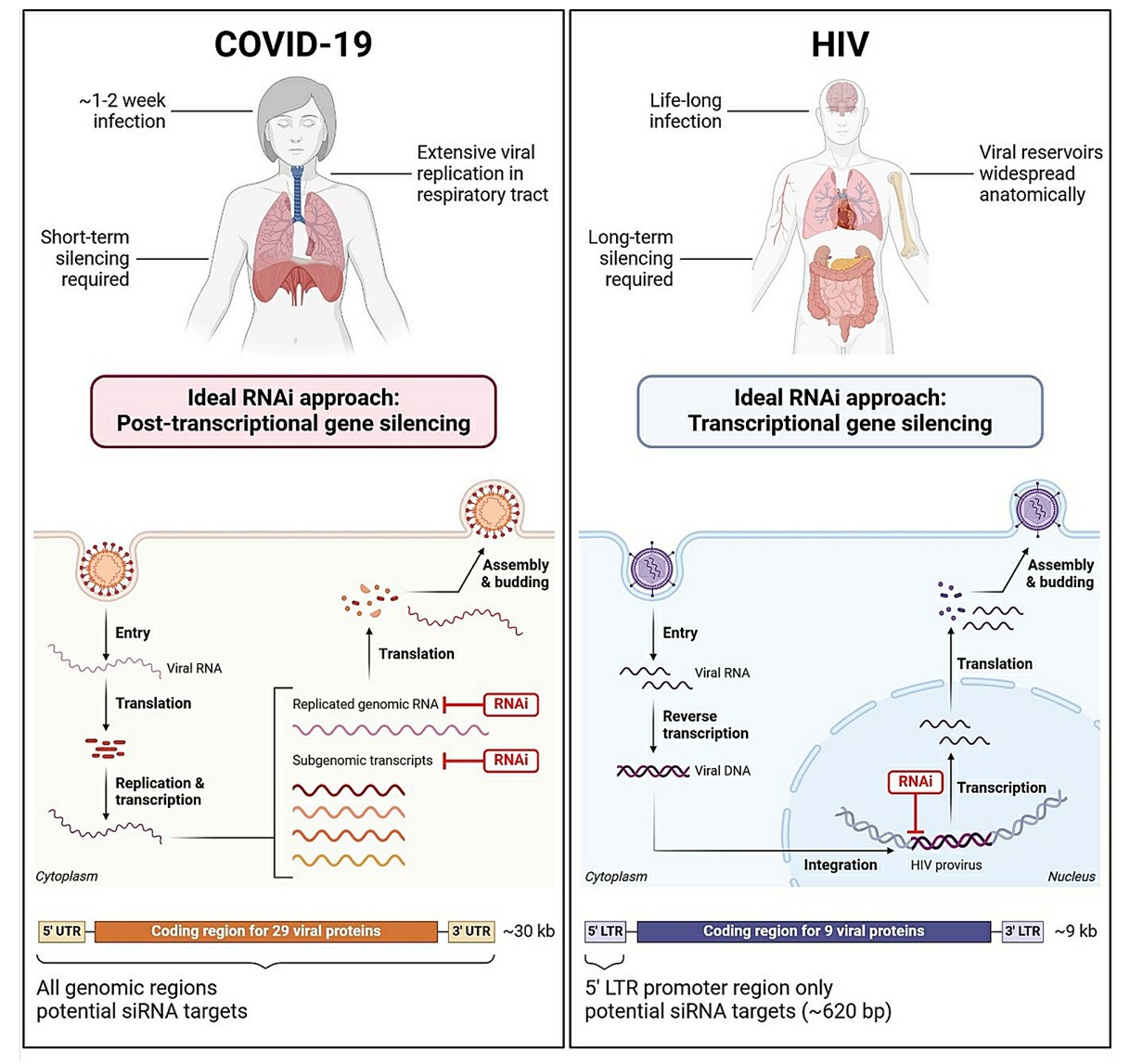 Fig.1 Differences between COVID-19 and HIV influencing RNAi approach and siRNA design strategy1,6.
Fig.1 Differences between COVID-19 and HIV influencing RNAi approach and siRNA design strategy1,6.
The Epigenetic Connection: siRNA's Hidden Talents
- siRNA-directed DNA methylation: Indirect genome editing
Studies indicate that siRNA directs DNA methylation which serves as an important epigenetic modification resulting in lasting alterations to gene expression. RdDM in plants enables siRNAs to guide DNA methyltransferases to specific genomic locations which results in new cytosine methylation. Various eukaryotic species maintain this mechanism which plays a critical role in controlling transposon activity and establishing gene imprinting patterns. The regulation of chromatin states in mammals has been linked with mechanisms that use siRNAs and Argonaute proteins (AGOs) yet the precise details remain ambiguous. The power of siRNA to guide DNA methylation shows that it can be used for indirect genome editing and maintaining long-term gene silencing.
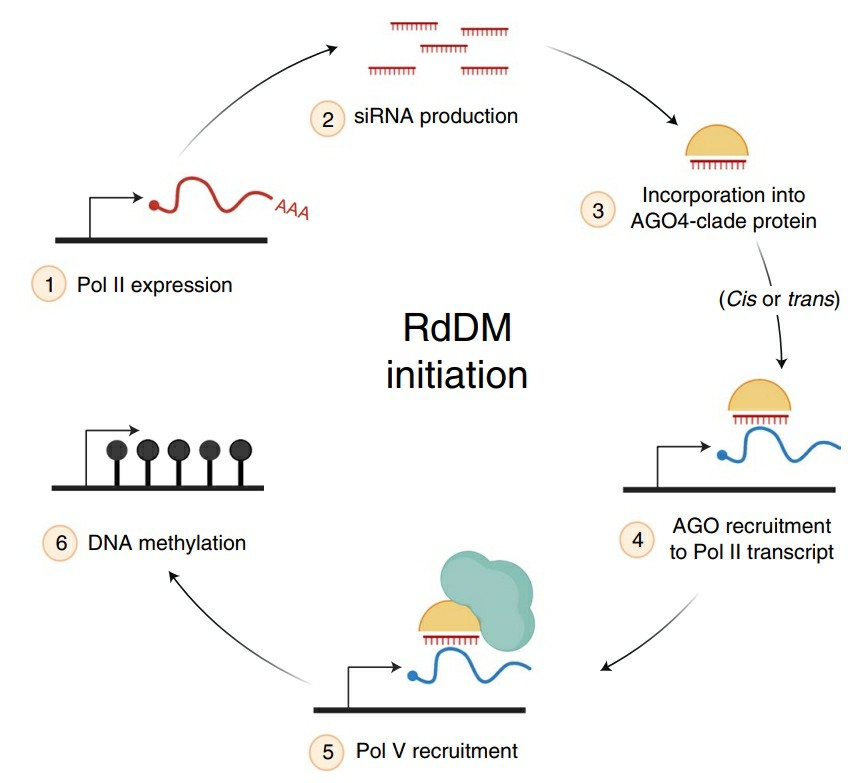 Fig. 2 Model of RNA-driven recruitment of Pol V to new RdDM targets2,6.
Fig. 2 Model of RNA-driven recruitment of Pol V to new RdDM targets2,6.
- Chromatin remodeling
Current research reveals that siRNAs perform chromatin remodeling which affects gene expression by altering chromatin structure. Research shows siRNAs direct histone-modifying enzymes to certain DNA areas which results in altered methylation and acetylation patterns of histones. Studies demonstrate that siRNAs trigger the dimethylation of histone H3K9 which typically signifies transcriptional repression. The mechanism changes chromatin accessibility which results in sustained alterations in gene expression patterns. Scientific research has modeled the interactions between siRNAs and chromatin states to demonstrate their dynamic interactions.
- Transgenerational effects: Emerging evidence in model organisms
Scientists have investigated the possibility of siRNA producing transgenerational effects through research conducted on multiple model organisms. siRNAs in plants trigger epigenetic changes which can be passed on to future generations and affect traits like gene expression patterns and stress responses. Studies have shown that in lower eukaryotes such as Schizosaccharomyces pombe siRNAs contribute to heterochromatin formation while also silencing repeated DNA sequences. Research indicates that epigenetic modifications induced by siRNA remain stable through inheritance and offer a way for traits to be passed on without genetic changes. Current research continues to explore transgenerational effects in mammals but demonstrates that siRNA has the capability to alter gene expression and phenotypes through multiple generations.
siRNA in the Brain: Conquering Neuroscience's Final Frontier
- Blood-Brain Barrier Breakthroughs: Novel Delivery Strategies
The selective permeability of the blood-brain barrier presents a major obstacle to siRNA delivery. Current developments in delivery methods demonstrate potential for breaking through this obstacle. Focused ultrasound and microbubbles make up one innovative method for scientific research. This approach utilizes focused ultrasound to temporarily break down the BBB so siRNA can access the brain. Scientists can design nanoparticles to pass through the BBB and send siRNA directly to desired brain cells for delivery purposes. The BCC platform utilizes intravenous injection to employ γ-secretase-mediated transcytosis for brain delivery of siRNA and other therapeutic compounds. The advancement of delivery approaches boosts siRNA transportation accuracy and effectiveness to brain cells which opens new treatment opportunities for neurological disorders.
- Neurodegenerative Diseases
Emerging research indicates siRNA as a potential therapeutic approach for neurodegenerative disorders including Alzheimer's and Parkinson's diseases. siRNA targeting BACE1 and APP genes demonstrates therapeutic effects in Alzheimer's disease through reduced amyloid plaque formation and enhanced cognitive function. Studies show that siRNA treatment which targets α-synuclein reduces the protein levels that contribute to Parkinson's disease pathology. Research evidence demonstrates that siRNA functions as a highly precise and effective therapeutic strategy for neurodegenerative disease treatment.
- Psychiatric Applications: Silencing Addiction-Related Genes
siRNA offers psychiatric treatment options by enabling researchers to target and suppress addiction-related genes. Research into siRNA-based targeting of addiction-related genes within reward circuits has led to new treatment strategies focused on the Drd2 gene. Experiments on animals demonstrate that silencing the Drd2 gene in the nucleus accumbens through siRNA decreases cocaine-seeking behavior. This method represents a groundbreaking addiction treatment strategy by precisely adjusting gene expression which affects addiction processes.
Agricultural Revolution: siRNA on the Farm
- Precision Pest Control: Targeting Pests with siRNA
The introduction of siRNA technology is transforming the agricultural sector by providing a specific and eco-friendly approach to pest management. Traditional pesticides harm beneficial insects and cause environmental contamination but siRNA-based solutions focus on specific pest genes which results in minimal effects on non-target species. The accurate targeting method of siRNA reduces the potential for pests to develop resistance and protects the ecological balance. Scientists have created siRNA sprays for crops that target vital genes in pests like aphids and whiteflies to effectively decrease pest numbers without damaging beneficial insects.
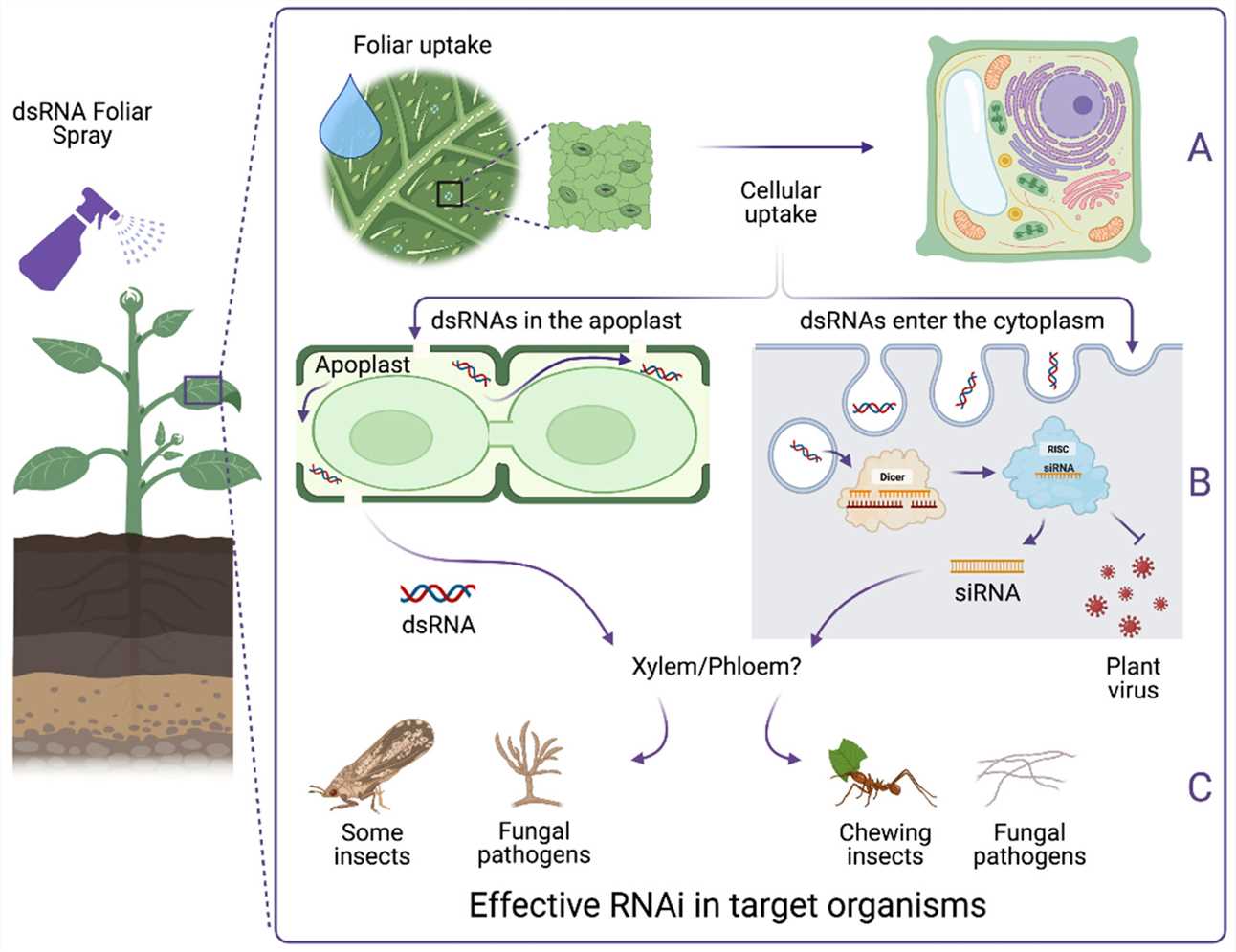 Fig.3 Functional crop protection via a foliar dsRNA spray to induce RNAi3,6.
Fig.3 Functional crop protection via a foliar dsRNA spray to induce RNAi3,6.
- Population Management: siRNA-Driven Gene Drives
Agricultural use of siRNA includes generating gene drives to control invasive species. Genetic constructs known as gene drives spread quickly through populations and when paired with siRNA they enable precise silencing of essential genes for invasive species survival and reproduction. This method shows promise for controlling pests that have become resistant to traditional pest management methods. SiRNA-based gene drives enable scientists to target reproductive genes in invasive species thereby decreasing their populations and lessening their harmful effects on native ecosystems.
- Nutritional Enhancement: Edible siRNA in GM Foods
The siRNA technology impacts pest management and enables the creation of genetically modified foods that offer enhanced nutritional value and health advantages. Scientists use edible siRNA to integrate siRNA molecules into plant crops which allows precise manipulation of gene expression. Scientists use this technology to deactivate genes that produce toxic substances while simultaneously increasing the expression of genes that generate beneficial nutrients. Researchers can engineer siRNA molecules to lower allergen production in cultivated plants which helps in creating safer foods for people who suffer from food allergies. Researchers can use siRNA to enhance the levels of essential vitamins and minerals which leads to better nutritional values in crops.
siRNA as a Diagnostic Tool
- Biosensor Innovation: siRNA for Real-Time Disease Detection
siRNA technology creates diagnostic advancements by developing innovative biosensors that enable real-time disease marker detection. The biosensors function by using RNA interference to attack and destroy particular mRNA sequences which are linked to different diseases. Researchers recently developed a siRNA-directed self-assembled quantum dot (QD) biosensor that enables simultaneous detection of multiple microRNAs at the level of individual particles. The biosensor utilizes quantum dots to report signals which allows for the simultaneous detection of multiple microRNAs in one sample with femtomolar sensitivity and single-base mismatch discrimination while eliminating the requirement for target labeling or amplification processes. The method delivers fast and precise diagnostics while reducing false positives and negatives which increases disease detection reliability.
- Integrated Theranostics: siRNA for Combined Diagnosis and Therapy
The combination of diagnostic and therapeutic capabilities into one medical platform represents an important development in healthcare technology and siRNA leads this progress. Theranostic methods merge siRNA's diagnostic features with precise therapeutic delivery systems. Engineered siRNA systems can release therapeutic agents in response to specific biomarker detection to ensure targeted treatment delivery. Dual-function medical technology delivers better disease management accuracy while minimizing systemic treatment side effects. The combination of siRNA-based diagnostic circuits and therapeutic modules enables systems to deliver complete solutions for detecting diseases and administering treatment which leads to better patient results and cuts down healthcare spending.
- Proactive Detection: siRNA for Early Cancer Diagnosis
These siRNA-based early warning systems advance cancer detection significantly by enabling proactive tumor identification during initial development stages. The utilization of siRNA to precisely target and degrade oncogenic mRNA sequences enables these systems to track cancer-related gene expression levels which serves as early tumor development indicators. Researchers can build siRNA circuits that identify elevated levels of oncogenes like KRAS or MYC which then activate therapeutic siRNA molecules to target and shut down these genes. The targeted approach improves cancer detection accuracy while simultaneously offering early tumor growth inhibition which may decrease the necessity for invasive treatments. Proactive disease management benefits from siRNA-based systems which improve cancer survival rates through early detection.
References
- Bowden-Reid, Ellen, et al. "Harnessing antiviral RNAi therapeutics for pandemic viruses: SARS-CoV-2 and HIV." Drug Delivery and Translational Research (2025): 1-22. https://doi.org/10.1007/s13346-025-01788-x.
- Sigman, Meredith J., et al. "An siRNA-guided ARGONAUTE protein directs RNA polymerase V to initiate DNA methylation." Nature Plants 7.11 (2021): 1461-1474. https://doi.org/10.1038/s41477-021-01008-7.
- Hoang, Bao Tram L., et al. "RNAi as a foliar spray: efficiency and challenges to field applications." International Journal of Molecular Sciences 23.12 (2022): 6639. https://doi.org/10.3390/ijms23126639.
- Bartels, Arthur, et al. "Dynamic DNA methylation in plant growth and development." International journal of molecular sciences 19.7 (2018): 2144. https://doi.org/10.3390/ijms1907214.
- Romero-López, Cristina, and Alfredo Berzal-Herranz. "siRNA Therapeutics: From Bench Lab. to Clinics." Pharmaceuticals 17.4 (2024): 416. https://doi.org/10.3390/ph17040416.
- Distributed under Open Access license CC BY 4.0, without modification.
