siRNA in Dermatology-Skinning the Surface
Introduction of siRNA in Dermatology
Small interfering RNA (siRNA) serves as an essential instrument for RNA interference (RNAi) which triggers gene silencing after transcription in eukaryotic cells. Because siRNA can precisely silence genes responsible for diseases dermatology has shown increased interest in this technology. siRNA therapy is best suited for the skin because its accessibility allows for extensive clinical trials targeting psoriasis and pigmented dermatosis. The main difficulty is transporting siRNA across the skin barrier which scientists solve through the creation of sophisticated transdermal delivery systems.
Introduction to Dermatological Disorders
- Overview of skin structure
The skin functions as a multilayered organ that defends the body against UV radiation and other external threats including trauma and microbial contamination. The skin consists of three primary layers which include the epidermis, dermis, and subcutaneous tissue that enable this tissue to exhibit high turnover capacity. The epidermis forms the body's outer skin layer which creates a waterproof barrier and is covered by a keratinized stratum corneum layer whereas the dermis represents a tough connective tissue beneath the epidermis housing hair follicles and sweat glands. Subcutaneous tissue forms the deepest skin layer which consists mainly of fatty and connective tissues. The skin performs several important functions for the body including immune monitoring of pathogens and maintaining body temperature while also allowing for sensory detection and managing bodily fluid loss. Skin repair can be achieved through various methods including autografts, allografts, tissue-engineered scaffolds and wound dressings. siRNA therapeutics present powerful alternatives to traditional skin regeneration techniques since they can easily access bodily tissues.
- Pathophysiology of dermatological disorders
Dysregulated immune systems and abnormal cellular communication processes define the pathophysiology of skin disorders. The interaction between T cells and cytokines including IL-17 and TNF-α along with keratinocytes results in chronic inflammation characteristic of psoriasis. Melanoma cells achieve uncontrolled proliferation and programmed cell death resistance through mutations in oncogenes and tumor suppressor genes such as BRAF and NRAS.
- siRNA as a Therapeutic Approach
Research using siRNA therapy for skin repair has shown effective outcomes in treating several skin conditions including psoriasis, allergic skin disease, epidermolysis bullosa simplex, epidermolytic palmoplantar keratoderma and pachyonychia congenita. A 1980 study demonstrated that specific growth factors activate keratinocyte migration which leads to skin regeneration. Transforming growth factor beta (TGF-β) which functions as a polypeptide cytokine helps regulate wound healing processes but scientists have yet to fully understand its precise role in wound healing. According to researchers Thy-1 siRNA decreases fibroblast cell migration while enhancing their proliferation by upregulating TGF-β1 expression in NIH3T3 cells. Research indicates that Thy-1 serves as an essential mediator for fibroblast cell movement and growth. Experiments validated the theory through testing both inside living organisms and in controlled lab environments. The pattern of Thy-1 localization reflects its gene function during early skin regeneration. The application of Thy-1 siRNA disrupted normal epithelialization and altered epidermal tissue structure surrounding wounds. The use of specific siRNA to silence the Thy-1 gene appears to influence the process of re-epithelialization during initial skin repair.
Mechanism of RNA interference in skin tissues
Dicer from the RNase III family cleaves double-stranded RNAs (dsRNAs) within cells into small RNA molecules called endogenous siRNAs. The processing mechanism for both endogenous and exogenous siRNAs becomes identical beyond this stage. Upon entering the cytoplasm siRNA integrates into the RNA-induced silencing complex (RISC) which is a multiprotein structure. AGO2 protein in the complex binds siRNA which results in splitting the siRNA duplex into single strands as the sense strand is removed while the antisense strand remains attached to AGO2. AGO2 and the antisense strand guided by RISC activation find and bind to their complementary mRNA target sequence through Watson–Crick base pairing. AGO2 breaks down the complementary mRNA into fragments after cleavage which stops it from being translated into proteins and blocks target gene expression to achieve gene silencing. The siRNA guide strand maintains its connection to the complex after cleavage which enables it to participate in successive cleavages of matching mRNA sequences.
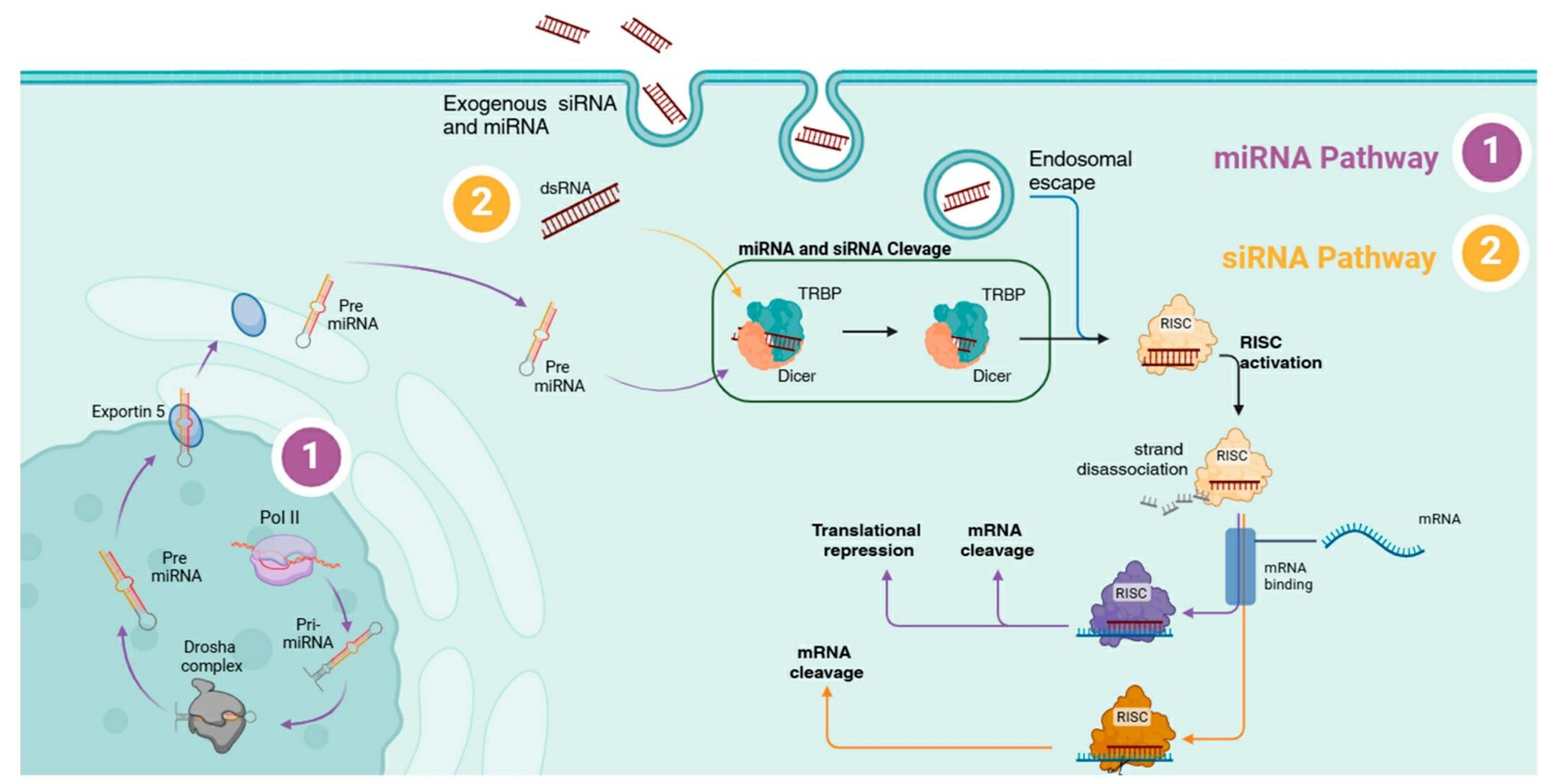 Fig.1 The RNAi mechanism of siRNA and miRNA1,6.
Fig.1 The RNAi mechanism of siRNA and miRNA1,6.
siRNA function in Skin Biology
- Targeting Genes Involved in Keratinocyte Proliferation
Control of keratinocyte proliferation is essential for skin biology but when this process becomes abnormal it creates various skin diseases like psoriasis. Psoriasis develops through skin cell accumulation since the production of keratinocytes surpasses their programmed cell death leading to plaque creation. siRNA targets and silences genes that regulate keratinocyte growth to restore proper skin function. Silencing the Fibroblast Growth Factor Receptor 2 (FGFR2) gene through siRNA application leads to decreased keratinocyte proliferation. When NFAT2 expression is suppressed it results in lowered keratinocyte proliferation and thinner epidermal layers. The application of siRNA to TRAF3 Interacting Protein 2 (TRAF3IP2) and Interferon Alpha Inducible Protein 6 (G1P3) genes successfully reduced cellular proliferation and boosted apoptosis rates. Studies show that siRNA regulates keratinocyte functions via gene silencing and offers an accurate treatment approach for skin conditions characterized by abnormal cell proliferation.
- Role of Genes like IL-17 and IL-23 in Psoriasis
Psoriasis emerges from persistent inflammatory responses created by interactions between immune cells and cytokines. IL-17 and IL-23 cytokines serve crucial functions in psoriasis development. IL-17 promotes keratinocyte growth along with inflammatory responses by enabling the NF-κB and JAK-STAT pathways. IL-23 sustains Th17 cell activation which leads to IL-17 secretion and perpetuates the inflammatory process The design of siRNA molecules enables precise targeting of these cytokines and their receptors which results in the interruption of the inflammatory sequence. Preclinical models demonstrated that siRNA directed against IL-17 effectively decreased both inflammation and keratinocyte proliferation. Blockage of downstream signaling pathways by using siRNA to silence the IL-23 receptor leads to reduced disease severity. siRNA therapy offers a precise and potent treatment possibility for psoriasis by targeting and modulating essential cytokines.
- siRNA Design for Targeting These Cytokines and Their Receptors
The process of targeting cytokines like IL-17 and IL-23 with siRNA requires selecting sequences that bind to the cytokine or receptor mRNA which results in the molecules being broken down. The creation of siRNA molecules requires detailed specifications that produce high specificity and effectiveness while minimizing unintended interactions. When siRNA targets IL-17A it results in decreased expression within keratinocytes which suppresses pro-inflammatory signaling pathways. Therapeutic siRNA which targets IL-23 receptor function suppresses Th17 cell activation and decreases IL-17 secretion. Scientists use advanced siRNA design techniques through chemical adjustments and delivery mechanisms to enhance siRNA stability and enable its transportation to skin target cells. The development of siRNA therapy for psoriasis requires these advancements to move from preclinical research stages into clinical applications.
- Inhibition of Oncogenes in Melanoma
The aggressive skin cancer known as melanoma develops from the abnormal functioning of oncogenetic components like BRAF and NRAS. Research indicates siRNA therapies that target specific oncogenes show promise in stopping tumor expansion and blocking metastatic spread. Melanoma displays sustained MAPK pathway activation due to BRAF mutations which results in enhanced cellular growth and survival. Using siRNA to target BRAF disrupts the signaling pathway which results in reduced tumor growth and boosts the efficacy of other treatments. siRNA molecules targeting NRAS can disrupt the RAS signaling pathway and inhibit melanoma progression. The combined silencing of BRAF and NRAS using siRNA treatment achieves a significant tumor size reduction alongside reduced metastatic potential in experimental melanoma models. Personalized treatment approaches through siRNA-based oncogene targeting can enhance therapeutic outcomes for melanoma patients.
- Impact on Tumor Growth and Metastasis
A thorough investigation of how siRNA-based oncogene silencing affects tumor growth and metastasis is essential for developing new melanoma treatments. siRNA attacks essential oncogenes such as BRAF and NRAS which helps hinder tumor development. siRNA treatment lowers BRAF expression which causes melanoma cells to stop growing and die due to MAPK pathway disruption. Tumor growth deceleration occurs alongside increased therapy susceptibility including immunotherapy and targeted treatment options. When NRAS is targeted using siRNA it blocks the RAS pathway which leads to decreased metastasis in melanoma cells. Preclinical studies with animal models of melanoma demonstrate that siRNA therapy diminishes tumor size and enhances survival rates. Research findings indicate that siRNA therapy presents a novel treatment approach for melanoma through precise and effective targeting mechanisms.
Challenges of siRNA therapy in Dermatological Research
- siRNA design and stability
The primary challenge for successful siRNA delivery through skin transdermal treatment involves maintaining siRNA molecular stability. When siRNA molecules penetrate the skin successfully they interact with numerous endogenous endonuclease enzymes that degrade them thereby reducing their therapeutic potency. Chemical modifications to siRNA molecules that change the sugar ribose structure and add methyl or thiophosphate groups to the phosphate backbone protect these molecules from nuclease degradation and lengthen their lifespan in the body. To achieve clinical applicability through enhanced protection against enzymatic degradation siRNA structures commonly undergo chemical modification of their ribose sugar by substituting the 2′-hydroxyl group with functional groups like 2′-O-methyl or phosphorothioate that resist nuclease action and improve siRNA binding affinity. The most advanced siRNA drugs in clinical development have adopted this strategy because it offers enhanced siRNA stability and silencing activity.
- Overcoming skin barriers
The skin's outermost layer called the stratum corneum makes the delivery of siRNA to the skin very difficult because it serves as both a physical and selective barrier against foreign substances. The skin's outermost layer contains hydrophobic structures and tight cell connections which prevent siRNA molecules from reaching deeper skin layers. The extracellular lipid array comprising nearly equimolar amounts of ceramides, free fatty acids, and cholesterol functions as a barrier against hydrophilic siRNA while maintaining skin water content. The dense network formed by tightly packed corneocytes and lipid-filled intercellular spaces creates additional barriers to siRNA penetration. Besides physical and chemical barriers, the SC's negative charge prevents negatively charged molecules such as siRNA from penetrating. The field of bioengineering and nanotechnology has developed several chemical and physical methods which demonstrate potential in breaking through the SC to allow siRNA delivery to deeper skin layers. The following methods have been developed to deliver siRNA through skin: (i) incorporation into complexes and nanocarriers, (ii) microneedle (MN) devices, (iii) iontophoresis, and (iv) fractional laser along with others.
- Intracellular barriers and endosomal escape
The success of siRNA delivery to skin cell action sites depends heavily on overcoming intracellular barriers. The siRNA or its vector must traverse the cell membrane of target skin cells to reach the cytoplasm where gene silencing can take place. Successful siRNA delivery needs thorough evaluation of several factors which include the charge of siRNA particles along with their hydrophobic characteristics and structural properties as well as cell membrane components such as glycosaminoglycans and charged lipids and receptors. Cell-penetrating peptides (CPPs) display exceptional transmembrane penetration ability while helping siRNA enter cells by creating non-covalent nanocomplexes through self-assembly and electrostatic siRNA interactions or by functioning on surfaces of siRNA-loaded nanoparticles. The following CPPs are undergoing investigation: TAT, penetratin (PNT), oligoarginine, transportan analogues, MPG, mellitin, poly-L-lysine, NickFect, PepFect, C105Y, CADY, KALA, and C6. The ability of CPPs to interact with membrane composition remains partially understood but involves multiple mechanisms. Glycosaminoglycans and receptors interact with CPPs through their hydrophobic domains and negatively charged groups to move across membranes. CPPs move through membranes using the transient-pore model together with the carpet-like model and inverted micelle model.
Apply nanoparticles in topical siRNA-therapy within dermatological research studies
- Liposomes
Since their discovery in the 1960s scientists have utilized liposomes as vesicular systems to encapsulate multiple molecules for delivery. These systems contain one or more bilayers that form spontaneously from phospholipids and cholesterol self-assembly which provide biocompatibility along with biodegradability and low toxicity while creating a suitable environment for molecules with various polarities. Liposomal systems represent a promising topical siRNA treatment option for skin conditions such as atopic dermatitis, psoriasis, and skin cancer. A liposomal formulation of siRNA Bcl-2 and paclitaxel delivered subcutaneously resulted in significant tumor growth reduction and inhibited cell proliferation in animal melanoma models. The outcome resulted from the combined downregulation of Bcl-2 by about 60% together with the activation of apoptosis through the decrease of pro-caspase-3 levels and increased Bax presence. The liposomal system features a cationic amphiphile built on a kojic acid backbone with an endosomal pH-sensitive imidazole ring enabling pH-dependent drug release within the tumor environment and endosomes (pH 5.5–6.5).
- Lipid nanoparticles
Lipid nanoparticles known as LNPs typically include cationic or ionizable lipids along with phospholipids, cholesterol and PEGylated lipids in their composition much like liposomal systems. Recent studies demonstrated that LNPs composed of cholesterol, DOTAP, and ethanol along with SPACE peptide decoration (attached to POPE-NHS lipid) enabled siRNA penetration into skin to increase by six times while achieving a 63% GAPDH protein knockdown in vivo. Researchers developed LNPs with DSPE-PEG and a cationic amphiphilic pyrrolidinium lipid (1,1-di-([Z]-octadec-9-en-1-yl)pyrrolidin-1-ium chloride) to temporarily fluidize the lipid matrix and improve transdermal siRNA delivery. These liposomes measured approximately 100 nm with a zeta potential of 30 mV that enabled enhanced skin penetration of siRNA reaching depths beyond 300 µm. Five-day topical treatment with LNPs containing siRNA TNFα and STAT-3 at a 50 µM concentration successfully decreased psoriasis area and severity scores in affected animals while molecular studies confirmed significant reductions in TNFα (approximately 10-fold) and STAT-3 (approximately 14-fold) gene expression resulting in decreased expression of downstream markers NF-kB (around 3.5-fold), Ki-67 (approximately 5-fold), and IL-23 (approximately 8-fold) compared to untreated diseased animals.
- Liquid crystalline nanoparticles
Mesogens self-assemble in water with stabilizers to create colloidal systems known as lyotropic liquid crystalline nanoparticles (LCNPs). Mesogens which are amphiphilic molecules naturally assemble into uni-, bi-, and three-dimensional supramolecular structures that demonstrate characteristics between fluid isotropic liquids and ordered anisotropic crystalline solids. Initial research found diamond-type bicontinuous cubic reverse hexagonal LCNPs with PEI or oleylamine (OAM) cationic agents displayed particle sizes between 160 and 230 nm alongside positive zeta potentials from 6 to 30 mV. The cationic agents PEI and OAM charged the LCNPs allowing efficient siRNA complexation which improved skin penetration and distribution during in vitro tests. The skin displayed higher siRNA fluorescence after treatment with hexagonal LCNPs because oleic acid functions as a penetration enhancer and exists in the hexagonal LCNPs while cubic LCNPs lack this fatty acid. The reverse hexagonal LCNP-PEI-siRNA GAPDH (10 µM) delivered topically led to successful protein knockdown at both 24 and 48 hours post-application; however, the reverse hexagonal LCNP-OAM-siRNA GAPDH only suppressed target protein levels after 48 hours.
Conclusions and future perspectives
The design and development of effective siRNA-based drug delivery systems requires a deep understanding of the skin tissue barrier structure and function along with its molecular targets for different pathologies. Medicine stands to benefit greatly from flexible therapies such as siRNA which can control autoimmune, inflammatory and neoplastic diseases by modulating target profiles that change during disease progression or show resistance to conventional methods. The latest research in basic and translational science has concentrated on creating siRNA vectors through organic and inorganic nanosystems while exploring physical methods to enhance siRNA bioavailability through the skin. Topical delivery barriers and cellular entry and endolysosomal escape can be overcome through surface modification with molecules that target specific sites or enhance cutaneous and cellular penetration. If researchers can solve these delivery barriers siRNA will become an effective tool for highly targeted and personalized medical treatments for skin diseases and other medical conditions.
References
- da Silva, Ualisson José, et al. "Nanotechnological approaches in topical RNAi therapy for skin diseases." Academia Biology 3.1 (2025). https://www.academia.edu/2837-4010/3/1/10.20935/AcadBiol7599.
- Sufianov, A.; Beilerli, A.; Kudriashov, V. Sufianov, Albert, et al. "Advances in transdermal siRNAs delivery: A review of current research progress." Non-coding RNA Research 8.3 (2023): 392-400. https://doi.org/10.1016/j.ncrna.2023.05.008.
- Zhang, Luyu, et al. "Novel pharmaceutical strategies for enhancing skin penetration of biomacromolecules." Pharmaceuticals 15.7 (2022): 877. https://doi.org/10.3390/ph15070877.
- Palmer, Brian C., and Lisa A. DeLouise. "Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting." Molecules 21.12 (2016): 1719. https://doi.org/10.3390/molecules21121719.
- Sun, Chenwei, Nitong Bu, and Xuefeng Hu. "Recent trends in electronic skin for transdermal drug delivery." Intelligent Pharmacy 1.4 (2023): 183-191. https://doi.org/10.1016/j.ipha.2023.08.001.
- Distributed under Open Access license CC BY 4.0, without modification.
