siRNA in Respiratory Diseases-Silencing the Respiratory Threats
Introduction of siRNA in Respiratory Diseases
Through RNA interference(RNAi), siRNA functions as a potential treatment option for respiratory conditions by silencing genes that cause disease. siRNA molecules with 20–25 nucleotides prevent disease-causing gene expression by binding to and degrading their target mRNA.. The targeted approach to therapy provides distinct benefits compared to traditional methods through its high specificity and potency as well as its capability to target previously "undruggable" targets. The pulmonary tissue delivery of siRNA enables non-invasive drug administration while the lung's low nuclease activity reduces drug degradation. The extensive surface area of alveoli enables enhanced drug absorption which allows for reduced local drug dosages and minimizes potential side effects. Lung epithelial cells stand as promising targets for respiratory disease treatment which makes inhalation therapy a crucial approach for lung conditions. The lung's anatomical structure along with its physiological functions and metabolic properties affect siRNA inhalation treatment outcomes which necessitates specialized drug delivery systems to achieve proper lung deposition.
Introduction to Respiratory Diseases
Overview of Respiratory Diseases
The respiratory system faces threats from several conditions which include infections as well as COPD together with asthma and interstitial lung diseases (ILDs). These diseases lead to extensive mortality rates worldwide while also causing global health crises. Respiratory infections caused by viruses and bacteria lead to significant economic costs and elevated rates of illness globally. COPD remains the primary cause of mortality globally while asthma continues to affect millions of people. Existing medical treatments for these diseases primarily address symptom management instead of curing the diseases. Most asthma treatment approaches utilize inhaled glucocorticoids and β2-receptor agonists yet these drugs fail to work effectively for some patients. Existing COPD therapies target symptom relief and life quality enhancement without tackling the fundamental disease processes. These treatment limitations demonstrate the necessity for developing new therapeutic methods.
Current treatment strategies and limitations
- siRNA as a Therapeutic Approach
siRNA holds promise as a respiratory disease therapy due to its precise targeting of crucial genes that drive these medical conditions. The siRNA molecule enters lung tissue cells and joins with the RNA-induced silencing complex (RISC) to initiate RNA interference. The binding of siRNA to RISC targets mRNA molecules for degradation which consequently blocks protein production. Targeted gene silencing works to lower the production of pro-inflammatory cytokines alongside viral genes and other elements that cause diseases. Preclinical model studies have shown significant antiviral effects from siRNA that targets the influenza viral polymerase complex. Asthma models have demonstrated beneficial effects from siRNA targeting TNF-α and IL-6 pro-inflammatory cytokines which resulted in reduced inflammation and better disease outcomes. SiRNA designed to target specific mRNA sequences provides a flexible treatment option that may be able to combat many different respiratory conditions.
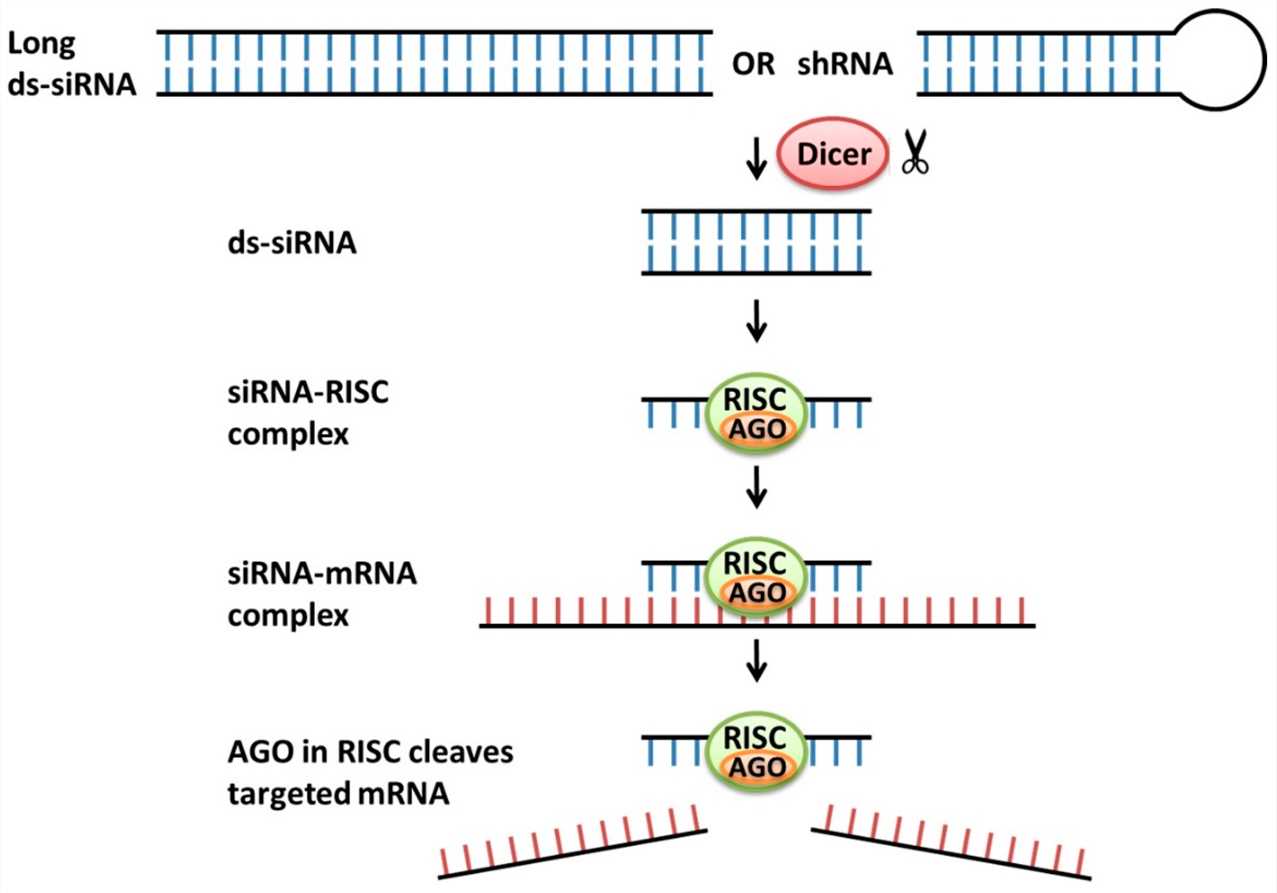 Fig.1 Mechanisms of actions of siRNAs1,6.
Fig.1 Mechanisms of actions of siRNAs1,6.
siRNA in Lung Biology
Targeting Genes Involved in Airway Inflammation
- Role of Cytokines in Asthma
The chronic inflammatory disease asthma affects airways through airway hyper-responsiveness (AHR), eosinophilic infiltration, reversible airflow obstruction, airway remodeling, mucus hypersecretion and goblet cell hyperplasia. The primary Th2 immune response in this disease arises due to cytokines IL-4, IL-5 and IL-13 production. The function of cytokines as critical components determines the pathophysiological processes of asthma. IL-4 induces B-cells to undergo IgE isotype switching which results in mast cell expression of high-affinity IgE receptor (FcεRI) that initiates allergic reactions and smooth muscle contraction. IL-5 causes eosinophils to migrate to the airways resulting in their accumulation which subsequently causes bronchial inflammation. The production of excessive mucus together with increased numbers of goblet cells driven by IL-13 leads to obstructed airways and respiratory symptoms.
- siRNA Design for Targeting These Cytokines and Their Receptors
Researchers can develop siRNA molecules to attach to the mRNA of particular cytokines and their receptors which results in decreased cytokine levels and reduced asthma-related inflammation. siRNA designed against IL-4 and IL-13 demonstrated substantial reductions of these cytokine levels in preclinical studies and resulted in diminished airway inflammation and better disease outcomes. Effective siRNA design requires the selection of sequences that match the target mRNA to achieve high precision while minimizing off-target effects. The silencing of IL-4 and IL-13 expressions along with their receptors by siRNA modulates Th2-driven inflammatory pathways that play a role in asthma.
- Inhibition of Mucus Production
Excessive mucus production is a frequent symptom among patients who have chronic airway diseases like asthma and COPD. When MUC5AC mucin production becomes excessive it leads to impaired mucociliary clearance which results in airway blockage along with respiratory symptoms. The MUC5AC mucin gets secreted mainly in the airways of asthmatic patients where it plays a key role in creating viscous mucus and mucus plugs.
- Impact on Airway Obstruction and Symptoms
siRNA therapy against MUC5AC demonstrates substantial promise in decreasing mucus output and enhancing airflow through the airways. Airway epithelial cells received MUC5AC-targeting siRNA through adeno-associated virus serotype 6 (AAV6) delivery from researchers. The AAV6 vector effectively transferred genetic material into epithelial cells of healthy mice airways and IL-13 stimulated human airway epithelial cells. AAV6-MUC5AC siRNA treatment reduced both MUC5AC mRNA and protein expression in IL-13-stimulated HAE cultures compared to untreated control samples. The AAV6-MUC5AC siRNA treatment preserved mucociliary transport rates in treated cultures which remained on par with those observed in healthy controls. The siRNA method targeting MUC5AC demonstrates effectiveness in reducing mucus hypersecretion and relieving airway blockage while improving respiratory symptoms in asthma patients.
Preclinical Research of siRNA for Respiratory Diseases
- siRNA in Mouse Models of Asthma and COPD
Researchers reported that the intranasal siRNA delivery of SOC3 to mice with chronic asthma resulted in lower eosinophil levels and reduced airways hyper-responsiveness. Studies have shown that this treatment resulted in better mucus secretion together with lower collagen levels and airway remodeling. siRNA targeting SOC3 blocks the RhoA/Rho kinase signaling pathway which leads to regulation of inflammation and bronchial smooth muscle contractions while enhancing IL-13 and IL-4 through RhoA protein interaction and STAT6 activation. This reduces cytokine expression and airways hyper-responsiveness. Research shows that IL-17 and IL-23 trigger neutrophilic and eosinophilic inflammatory responses in mice. The process of silencing SOC3 through siRNA affects IL-17 expression levels.
Heightened mucus production and inflammation from ambient particulate matters (PMs) lead to respiratory diseases such as asthma and chronic obstructive pulmonary disease. A research team in 2019 investigated how Amphiregulin (AREG), which binds to the epidermal growth factor receptor (EGFR), functions in inflammation and increased mucus production when human bronchial epithelial cells encounter ambient particulate matter (PM). AREG-siRNA treatment led to significant suppression of PM-induced inflammation and mucus hypersecretion. The treatment also inhibited the activation of the EGFR-AKT/ERK signaling pathway.
- siRNA in Viral Respiratory Infections
The application of siRNA demonstrates considerable promise for neutralizing viral genes responsible for respiratory diseases including influenza and COVID-19. siRNA directed at polymerase genes like PB1, PB2 and PA within influenza models shows strong antiviral effects through suppression of viral replication. Research using COVID-19 models demonstrated that siRNA aimed at the SARS-CoV-2 spike protein together with other genomic regions reduces viral load while also decreasing disease severity. siRNA-mediated antiviral defense functions through the RNAi pathway. siRNA molecules direct the RISC to destroy viral mRNA which blocks viral protein synthesis and replication. Through targeting strategies scientists can lower viral titers and safeguard host cells against infection.
- The application of siRNA in mouse models for lung cancer research
Scientists created nanostructured lipid-based carriers (NLCs) that enable simultaneous delivery of an anticancer medication and siRNA designed specifically for lung cancer therapy. The nanocarriers containing drugs trigger cell death while siRNA counters multi-drug resistance. The research included two siRNA delivery forms to increase treatment efficiency where the first siRNA attacked the MRP1 (Multidrug resistance-associated protein) mRNA to stop the main drug efflux transporter. The second siRNA attacked Bcl2 (B-cell lymphoma) mRNA to disrupt the cell's anti-apoptotic defense system. Patients received lung-targeted drug treatment through inhalation after the drug was encapsulated in NLCs. Nanocarriers accumulate mainly in lungs through inhalation while intravenous injection results in greater accumulation in liver, spleen, and kidney compared to lungs. The engineered NLC formulation transported the drug and siRNA directly into cancer cells. Through targeted gene silencing researchers achieved induced cell death in lung tumor cells.
Delivery system of siRNA in the respiratory system
siRNA displays significant potential for both treating and preventing a variety of lung infections. The RNA particles successfully enter human targets which may then block specific gene series via RNA interference mechanisms resulting in therapeutic effects. Successful siRNA treatment translation from research labs to clinical settings faces major obstacles due to delivery challenges. An ideal delivery operator must protect siRNA from enzymatic degradation while facilitating cellular uptake and promoting endosomal escape inside cells without causing harmful effects. Basic delivery methods or lung delivery techniques can be used to achieve pulmonary targeting. The second delivery method might provide improved siRNA preservation in lung tissue while reducing essential toxic effects. The delivery design needs careful planning to enhance siRNA deposition in the unhealthy areas of the aviation routes. During lung siRNA treatment studies conducted in vivo most researchers delivered siRNA through intranasal or intratracheal routes.
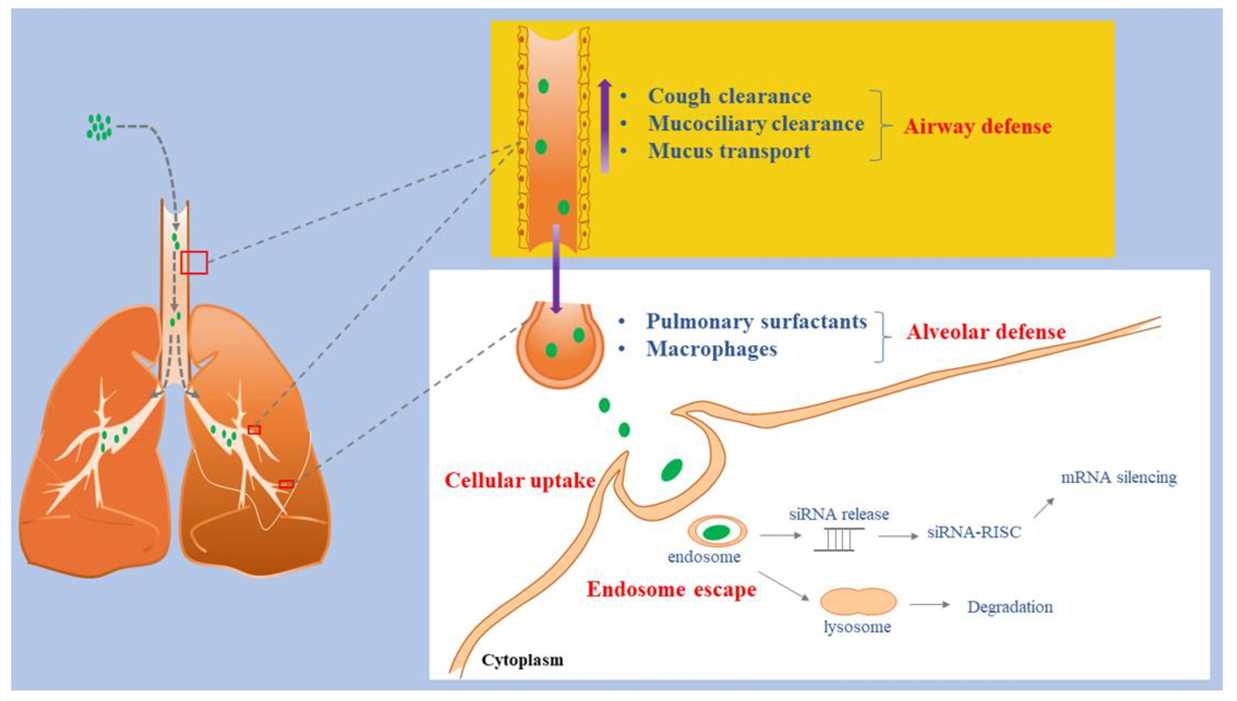 Fig.2 Schematic illustration of siRNA inhalations delivery barriers2,6.
Fig.2 Schematic illustration of siRNA inhalations delivery barriers2,6.
- Lipid-based delivery vectors
For both laboratory and animal studies researchers commonly employ lipid-based systems to administer siRNA. Negatively charged siRNA interacts with cationic lipids or liposomes using electrostatic forces to create structures known as lipoplexes. A number of commercially available delivery agents are lipid-based and some are specifically designed for in vivo lung delivery such as DharmFECT, lipofectamine, and Oligofectamine. Clinical application of lipid-based delivery vectors faces challenges due to their toxic effects and their ability to trigger non-specific inflammatory cytokine and interferon responses. Lipid-based delivery vectors can be classified into five types of molecules: Lipid-based delivery vectors fall into five distinct molecular categories namely Cationic lipoplexes and liposomes as well as PEGylated lipids which together with Neutral lipids then Lipids particles and Lipid-like molecules make up the complete classification.
- Polymer-based delivery vectors
Delivery vectors based on polymers possess adaptive properties that allow their physicochemical characteristics to adjust efficiently to meet various delivery needs. Studies indicate that polymers generally do not trigger significant immune system reactions. The two main categories of polymer-based vectors include polycations and polymeric nanoparticles. Engineered polycations like polyamidoamine dendrimers and polyethyleneimine alongside natural polycations such as chitosan have served to deliver DNA for many years.
- Peptide-based delivery vectors
The discovery of the TAT protein from HIV-1 which enhances viral entry into cells led to the identification of multiple cell-penetrating peptides (CPPs). Cell-penetrating peptides are predominantly used to deliver restorative macromolecules. Scientists employ this method to transfer siRNA into cells. Researchers have evaluated several CPPs and their derivatives as potential vehicles for delivering siRNA including MPG, TAT, transportan, penetratin, CADY, and LAH4. The connection between peptides and siRNA happens through either covalent disulfide bonds or electrostatic noncovalent interactions.
Future directions in siRNA-based therapies
siRNA-based therapies offer promising prospects as research continues to solve critical issues while finding new applications. The transfer of preclinical findings to clinical applications faces significant obstacles because researchers lack a comprehensive understanding of disease mechanisms and suitable animal models. Research during preclinical stages has mainly concentrated on blocking inflammatory cytokines but the success levels in gene silencing and therapeutic outcomes have been inconsistent. siRNA applications could benefit from combination therapy approaches. Research on siRNA applications for respiratory diseases has tested its combination with corticosteroids or bronchodilators through preclinical models. LNPs delivered siRNA that targets IL-4 and IL-13 cytokines in asthma models which led to diminished airway inflammation alongside enhanced lung function. The use of siRNA to target genes responsible for inflammation and tissue remodeling in COPD models has demonstrated potential to lessen the severity of the disease. The research demonstrates that siRNA can serve as an effective treatment option for respiratory diseases when combined with current therapeutic agents. Preclinical model case studies demonstrating successful combination therapies highlight the therapeutic promise of siRNA. Researchers successfully optimized a lipidoid-polymer formulation for siRNA encapsulation against TNF-α which resulted in optimal gene silencing in macrophages.
References
- Liao, Wupeng, et al. "Oligonucleotide therapy for obstructive and restrictive respiratory diseases." Molecules 22.1 (2017): 139. https://doi.org/10.3390/molecules22010139.
- Fan, Yulin, and Zhijun Yang. "Inhaled siRNA formulations for respiratory diseases: from basic research to clinical application." Pharmaceutics 14.6 (2022): 1193. https://doi.org/10.3390/pharmaceutics14061193.
- Mehta, Aditi, Thomas Michler, and Olivia M. Merkel. "siRNA therapeutics against respiratory viral infections—What have we learned for potential COVID‐19 therapies?." Advanced healthcare materials 10.7 (2021): 2001650. https://doi.org/10.1002/adhm.202001650.
- Shrestha, Jesus, et al. "Advanced models for respiratory disease and drug studies." Medicinal Research Reviews 43.5 (2023): 1470-1503. https://doi.org/10.1002/med.21956.
- Zhang, Yuan, et al. "Nanoparticle delivery platforms for RNAi therapeutics targeting COVID-19 disease in the respiratory tract." International journal of molecular sciences 23.5 (2022): 2408. https://doi.org/10.3390/ijms23052408.
- Distributed under Open Access license CC BY 4.0, without modification.
