Applications of Gene Therapy in Cancer Treatment
Current Status of Cancer Treatment
Modern cancer treatment strategies show substantial variation because they target different aspects of the disease. Throughout many years traditional cancer treatments such as surgery alongside chemotherapy and radiation therapy remained the main medical solutions. Traditional cancer treatments generally fail to provide precise treatment and cause substantial side effects together with limited therapeutic success. Chemotherapy functions by attacking rapidly dividing cells but it also harms healthy cells which leads to side effects including hair loss, nausea and fatigue. Radiation therapy delivers successful results against localized tumors but simultaneously damages adjacent healthy tissues. Current developments in immunotherapy reveal its potential as an effective cancer treatment option through the deployment of natural immune system defenses. Checkpoint inhibitors-based therapies show strong results in some patients but do not work for everyone and create significant side effects. Targeted therapies that identify and attack molecular markers in cancer cells have improved treatment outcomes for specific cancer types. Traditional medical treatments struggle to manage cancer effectively when resistance develops. Gene therapy attracts research attention because of its promising potential as a treatment method.
What is a Gene Therapy
Medical treatment through gene therapy involves using genetic material to prevent diseases and provide therapeutic solutions. Scientists insert modified genes into cells to replace defective genes and create therapeutic proteins. The primary goal of gene therapy is to correct harmful genetic mutations which lead to diseases like cancer through modifications to cellular genetic material. Gene therapy utilizes multiple techniques to achieve its goals including viral vectors for new genetic material delivery, CRISPR-Cas9 for accurate gene modifications and RNA interference (RNAi) to suppress detrimental genes. Gene therapy delivery methods include direct tissue administration alongside intravenous infusion as well as ex vivo techniques that modify cells outside the body for subsequent reintroduction. Advances in molecular biology along with genetic engineering and delivery technologies have made gene therapy a fast-growing field which holds great potential in medical science. Gene therapy continues to evolve through its developmental phase across numerous applications yet demonstrates substantial promise in treating various genetic disorders and specific cancer types.
Importance of Gene Therapy in Cancer Treatment
Gene therapy offers groundbreaking possibilities for cancer treatment through its ability to address disease origins at the genetic and molecular levels. Traditional therapies attack rapidly dividing cells but gene therapy precisely targets the underlying genetic mutations responsible for cancer development. Therapies become more efficient when they achieve precision because they produce potentially fewer adverse effects. Certain genetic mutations in some cancers result in the activation of oncogenes and the suppression of tumor suppressor genes. Through gene therapy scientists can repair genetic mutations which restores healthy cell function and stops cancer development. Scientists consider the application of CRISPR-Cas9 gene-editing technologies as one of the most promising methods for treating cancer through gene therapy. These tools enable precise DNA modifications within cancer cells that may remove harmful genetic mutations which cause cancer. CRISPR technology enables scientists to modify cancer cell genomes to increase immune system targeting and improve immunotherapy outcomes. Gene therapy delivers therapeutic genes straight to cancer cells which then produce proteins that block tumor expansion and trigger cancer cell death.
Basic Principles of Gene Therapy
- Definition and Mechanisms of Gene Therapy
Gene therapy operates as a medical process where genetic material is delivered into a patient's cells with the goal of treating or curing diseases. The cutting-edge method remedies genetic diseases by replacing defective genes with their functional counterparts or by editing them. The mechanisms of gene therapy can be broadly categorized into three main approaches: gene addition/augmentation, gene suppression, and genome editing.
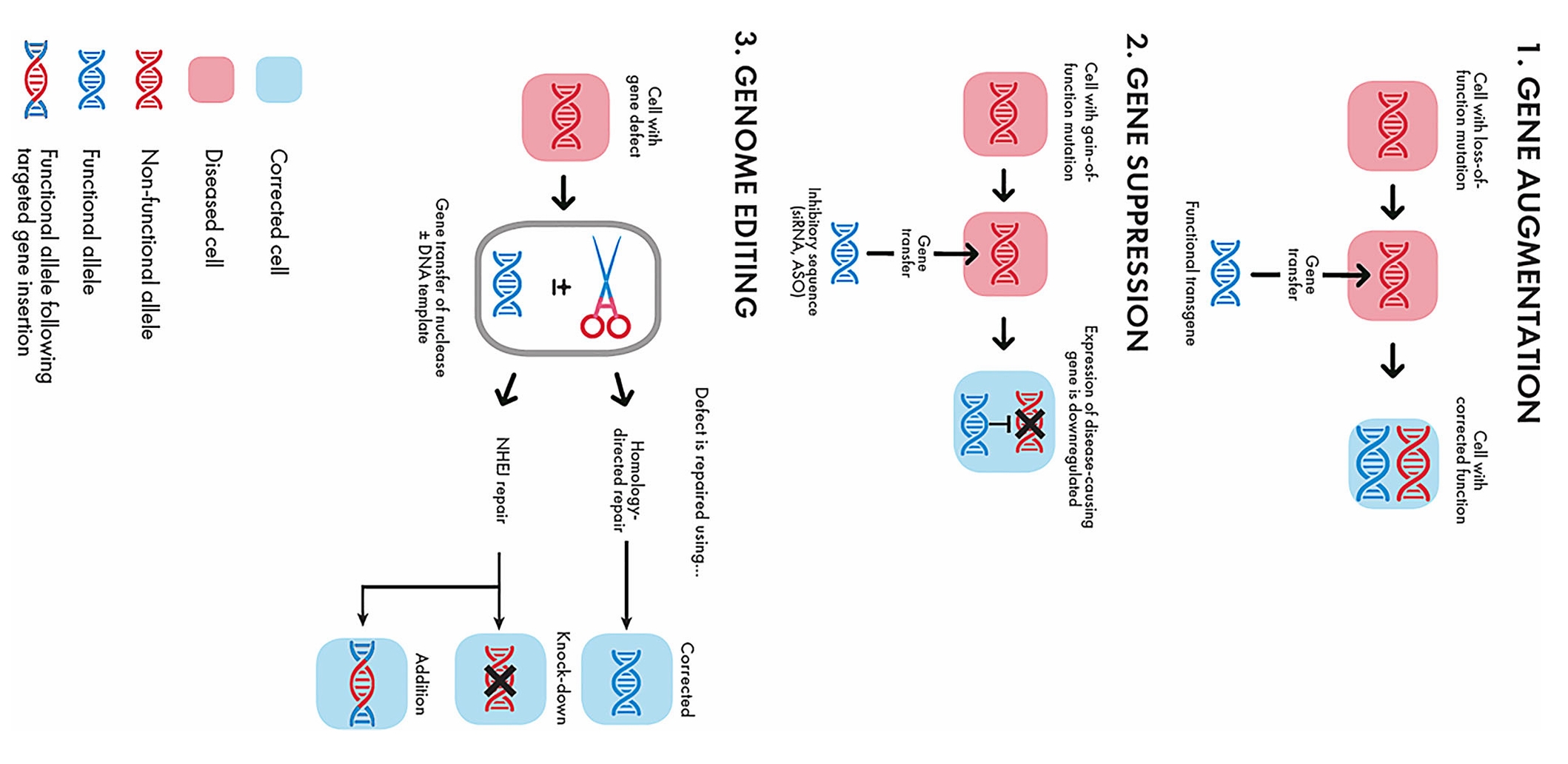 Fig. 1 Types of gene therapy: gene augmentation, gene suppression, and genome editing1,6.
Fig. 1 Types of gene therapy: gene augmentation, gene suppression, and genome editing1,6.
- Gene Addition/Augmentation
Medical professionals use this treatment method when a disease results from a gene's functional loss. When patients suffer from genetic loss of function diseases, their cells receive an additional functional gene copy to reestablish normal cellular operations. Researchers deliver a functional version of the defective gene to lung cells in genetic disorders like cystic fibrosis to repair the genetic abnormality.
- Gene Suppression
Gene therapy targeting gain of function diseases includes the suppression of harmful genes when they become overactive and cause cancer. Doctors achieve gene suppression by inserting inhibitory sequences like microRNAs or short hairpin RNAs into target cells to lower the expression levels of the problematic gene.
- Genome Editing
The genome editing method serves as a precise and advanced tool that enables direct alterations to the DNA of patients. Scientists use CRISPR-Cas9 tools to conduct accurate genetic repairs targeting individual base-pair changes in DNA sequences. The method lets researchers fix damaging genetic mutations responsible for diseases including sickle cell anemia and β-thalassemia. CRISPR technology allows scientists to modify cancer cell genomes in a way that makes them more vulnerable to immune response thus improving immunotherapy treatment results.
Goals of Gene Therapy
Through the replacement of defective genes with functional ones or by correcting mutations gene therapy brings back normal cell activity and reduces disease manifestations. The treatment method of gene therapy introduces correct genes which replace faulty or missing ones to halt disease advancement in single-gene disorders like spinal muscular atrophy. A primary goal of gene therapy involves stopping genetic diseases from advancing before permanent damage sets in. Gene therapy that begins early in the treatment process can halt disease advancement and enhance patient well-being. Through gene therapy patients receive individualized medical treatments which target their unique genetic mutations. Patients experience better treatment results while also facing lower chances of resistance development compared to typical standard therapies using this approach. Improved therapeutic results are achieved through the combination of gene therapy with chemotherapy, radiation therapy, and immunotherapy treatments. Gene therapy treatment makes cancer cells vulnerable to immune system attacks which increases the efficacy of immunotherapy.
Types of Gene Therapy Modalities in Oncology
- Gene Replacement Therapy
During gene replacement therapy scientists introduce normal gene versions into cancer cells to fix missing or faulty genes thereby restoring proper cell functionality. This therapeutic approach provides significant advantages to cancer patients who have genetic alterations like Tumor Protein 53 (TP53). The therapeutic adenoviral vector Gendicine delivers the p53 gene to treat head and neck squamous cell carcinoma effectively. This accomplishment stands as a significant development in cancer gene therapy progression. New developments in adeno-associated viral (AAV) vectors have enhanced both the safety and accuracy of gene replacement treatments which broadens their applicability to various cancer types including solid tumors such as breast, lung and colon cancers. The therapies administer therapeutic genes to tumors using AAV viral vectors which may produce sustained responses and functional cures in some instances.
- Gene Silencing Therapy
Gene silencing therapy serves as a critical cancer treatment approach by inhibiting oncogene expression which drives cancer development. RNAi therapy has become significant in contemporary medical treatments because recent chemical and delivery system enhancements allowed regulatory approval of multiple siRNA-based medications. Current advancements have strengthened RNAi therapies by providing both increased stability and effectiveness while reducing off-target effects. The RNA interference process utilizes siRNA and shRNA molecules to degrade mRNA which stops the production of defective proteins. Clinical trials for pancreatic and liver cancer patients aim to target PLK1 using siRNA. Early research on aggressive solid tumors shows that RNAi-based treatments may be effective. siRNA and miRNA-based gene silencing treatments show strong potential in inhibiting oncogenes that drive tumor progression. siRNA molecules attach to specific mRNA sequences which stops the production of proteins that encourage cancer cell growth. Various cancer types have seen researchers use this gene-deactivation technique to target progression-enhancing genes. RNAi-based therapies demonstrate potential for oncogene targeting and operate alongside established gene-editing methods such as CRISPR. Recent miRNA therapy research demonstrates significant progress against colon and lung cancer by silencing oncogenes and boosting tumor suppression functions.
- Oncolytic Virotherapy
Oncolytic virotherapy uses genetically modified viruses to destroy cancer cells without harming normal cells. The FDA gave approval to Imlygic (Talimogene laherparepvec) as an oncolytic virus which demonstrated therapeutic effectiveness in melanoma treatment. Cancer cells experience destruction through oncolytic virus infection followed by cellular breakdown which triggers immune system attacks on leftover cancer cells. Current research efforts focus on applying oncolytic viruses to solid tumors such as prostate and pancreatic cancers while improving their tumor infiltration capabilities and immune system activation. New research reveals that the combination of oncolytic virotherapy with immune checkpoint inhibitors greatly increases immune response effectiveness against solid tumors that use immune evasion strategies and have complex tumor microenvironment (TME).
- Suicide Gene Therapy
Suicide gene therapy consists of delivering genes to cancer cells that transform safe prodrugs into deadly agents which kill tumor tissue cells specifically. The Herpes Simplex Virus Thymidine Kinase (HSV-TK) gene represents one of the most established methods which activates the antiviral drug ganciclovir through phosphorylation to form a toxic compound that destroys tumor cells during cell division. Clinical trials indicate this method demonstrates effectiveness for difficult-to-treat cancers like gliomas and pancreatic cancer. The HSV-TK/ganciclovir system demonstrates effectiveness in shrinking glioma tumors because the bystander effect eliminates both genetically altered cells and nearby tumor cells. Cytotoxic metabolites move from treated cells to neighboring cells which results in better therapeutic results. This combination therapy approach has produced positive outcomes because the conventional radiotherapy treatment enhances tumor cell sensitivity to prodrug cytotoxic effects.
- Autologous Chimeric Antigen Receptor (CAR)-T Cell Therapy
The development of CAR-T therapy has progressed substantially since its beginning. The initial CAR-T cell design incorporated just one signaling domain (CD3ζ) which functioned mainly to activate T cells. The second-generation CAR-T cells gained improved T cell expansion and persistence through the addition of costimulatory domains like CD28 or 4-1BB. The third generation of CAR-T cells enhanced T cell functionality by integrating multiple costimulatory domains together. Fourth-generation CAR-T cells known as TRUCKs (T cells redirected for antigen-unrestricted cytokine-initiated killing) improved immune responses within the TME by using cytokine signaling including Interleukin-12 (IL-12). The fifth generation added advanced signaling pathways specifically designed to improve tumor targeting and neutralize immunosuppression effects from the TME. Autologous CAR-T cell therapy requires doctors to extract T cells from patients before they genetically modify these cells to create a CAR-T targeting tumor antigens and then reintroduce the engineered cells into the patient. Advanced personalized immunotherapy transformed the way hematologic cancers are treated by achieving remarkable outcomes in patients with recurring or resistant B-cell malignancies including leukemia and lymphoma.
- Allogeneic CAR-T Cell Therapy
Despite its proven effectiveness against specific cancers autologous CAR-T therapy faces important limitations including extended production periods high financial costs and variability between patients. Patients with histories of comprehensive treatments or compromised immune systems face greater difficulties with these challenges. The "off-the-shelf" CAR-T therapy known as allogeneic CAR-T therapy presents an encouraging solution to these treatment limitations. Donor T-cells are genetically modified to express CARs while receiving alterations that prevent immune rejection in the recipient through this therapeutic approach. Allogeneic CAR-T therapy grants an advantage as it allows for manufacturing CAR-T cells beforehand which ensures immediate availability for multiple patients. The treatment method tackles scalability obstacles and enhances patient accessibility compared to individualized autologous treatments. Allogeneic CAR-T cells become a practical treatment option for patients who experience weakened immune systems or have insufficient healthy T-cells because of prior treatments or additional health complications that render autologous therapies infeasible. Patients with aggressive cancers require immediate treatment which necessitates faster production times for allogeneic CAR-T cells because the manufacturing timeline for autologous therapies extends too long.
- Epigenetic Modification in Gene Therapy
Epigenetic modifications play a crucial role in cancer development by modifying gene activity without DNA sequence changes through oncogene activation and tumor suppressor gene silencing along with immune evasion processes. The CRISPR/dCas9 systems represent cutting-edge epigenetic tools that enable cancer treatment through reversible gene expression regulation without DNA sequence modification. The modified CRISPR-Cas9 system known as CRISPR/dCas9 binds specific DNA regions to either activate (CRISPRa) or silence (CRISPRi) gene expression without cutting DNA. CRISPR/dCas9 technology enables precise control of oncogenes and tumor suppressors through epigenetic modifications without genome alteration by binding effector proteins that change DNA methylation or histone status. Oncology benefits from the ability to adjust gene expression levels because this leads to improved therapeutic results. CRISPR/dCas9 system can work on multiple genes at once to regulate oncogenic pathways and turn on tumor suppressor genes. The multi-target strategy efficiently addresses tumor heterogeneity which leads to more sustainable treatment results.
Delivery Methods in Gene Therapy
The method used to transport therapeutic genes to target cells plays a crucial role in the effectiveness of gene therapy approaches. Gene therapy delivery systems are classified into four main categories: viral vectors and non-viral vectors as well as physical methods and live biotherapeutic products (LBPs). Every delivery system comes with unique advantages and limitations while scientists continue to refine these systems for safer and more effective clinical use.
- Viral Delivery Systems
Adenoviral (AdV) vectors
AdV vectors deliver genes well to non-dividing cells but trigger powerful immune reactions. Engineering advancements are being pursued to address these effects and improve safety for clinical use by developing helper-dependent adenoviral vectors that contain no viral coding sequences which decrease immunogenicity and extend transgene expression duration.
Adeno-associated viral (AAV) vectors
AAV vectors demonstrate high efficiency while maintaining safety through their minimal immunogenicity. Researchers commonly apply these vectors in both in vivo and ex vivo gene therapy settings to treat a variety of conditions from hemophilia to cancer. Pre-existing immunity to AAV together with its limited payload capacity create significant obstacles. Overcoming AAV vector challenges involves creating new AAV serotypes and engineered capsids to avoid neutralizing antibodies and using self-complementary AAV vectors to boost transgene expression within the constraints of limited packaging capacity.
Lentiviral vectors
Lentiviral vectors originate from HIV and can stably integrate into the host genome which allows for lasting gene expression. Gene therapy applications such as CAR-T cell treatments and stem cell engineering rely heavily on these vectors. The ability of these vectors to deliver genetic material to both dividing and non-dividing cells combined with their significant packaging capacity makes them highly effective instruments for gene therapy. Current studies aim to enhance vector safety by developing self-inactivating vectors and adding insulator elements to block insertional mutagenesis.
- Non-Viral Systems
Lipid nanoparticles (LNPs)
Lipid nanoparticles (LNPs) became widely recognized during the COVID-19 pandemic because of their role in mRNA vaccine distribution but researchers are now evaluating their potential for gene therapy applications. LNPs package nucleic acids and therapeutic elements which allows for secure transport with decreased immune system activation. The use of CRISPR-Cas9 delivery systems shows great potential in treating cancer.
Polymeric nanoparticles
Synthetic polymers possess the ability to transport genes and small interfering RNA (siRNA) as well as other molecular entities into cellular environments. Functionalizing ligands to target therapeutic Mesenchymal stem cells (MSC) constructs will increase tumor tissue concentration and improve antitumor effectiveness. Recent research efforts aim to enhance targeting precision while minimizing unintended effects which establishes these systems as essential instruments for solid tumor treatments.
Exosome-mediated delivery
Exosomes function as naturally occurring extracellular vesicles which provide a precise and immunologically safe way to transport therapeutic substances. These cell-derived vesicles have built-in targeting functions which positions them as effective tools for gene delivery in cancer treatment. The latest research shows how exosomes can deliver genetic materials like mRNA, siRNA, and CRISPR components directly to cancer cells even inside the difficult Tumor Microenvironment. Exosome delivery systems offer significant benefits by avoiding many obstacles encountered by alternative delivery methods which include immune system activation and inadequate penetration through solid tumors.
Progress in Preclinical Research of Cancer Treatment
- Animal Model Studies
Scientists use animal models to evaluate how CRISPR-Cas9 can repair mutations that lead to cancer. Researchers used CRISPR to modify mouse cancer cell genomes by reactivating tumor suppressor genes and disabling oncogenes. Research outcomes indicate CRISPR-Cas9 applications have effectively decreased tumor expansion while increasing patient survival time. Researchers use animal models to determine how well viral vectors like adenoviral vectors and lentiviral vectors deliver therapeutic genes to cancer cells while checking their safety. Research shows AAV vectors successfully transport anti-cancer protein-encoding genes to mouse tumors which results in significant tumor shrinkage.
- Cell Culture Experiments
Researchers test gene-editing tools such as CRISPR-Cas9 for efficiency and accuracy through cell culture experiments. Research indicates that CRISPR is effective at repairing mutations in cancer cell lines which restores gene functionality and prevents tumor cell proliferation. Research with cell cultures plays a vital role in detecting and reducing unintended effects produced by gene-editing technologies. Next-generation sequencing (NGS) analyzes treated cell genomes to detect accidental mutations from CRISPR treatments. Through these studies researchers can enhance gene-editing tools by boosting their specificity along with ensuring their safety.
Challenges and Solutions
- Safety and Efficacy Issues
Targeted delivery
The effectiveness of therapeutic gene delivery depends on the precision targeting of cancer cells. The natural tropism of viral vectors used in gene therapy creates challenges because it results in the non-specific uptake of these vectors by non-targeted cells. Scientists are creating new methods such as trans-ductional retargeting which involves altering viral surface proteins to specifically attach to cancer cell receptors. Scientists have altered adenoviral vectors to produce specific ligands which bind to tumor receptors because this modification increases their infection rate in cancer cells and decreases unintended infections in other cells.
Vector safety
The safety of viral vectors represents a primary concern. When viral vectors are taken up by organs like the liver or spleen rather than cancer cells, they can cause unwanted side effects and lose their effectiveness. Researchers are exploring innovative delivery approaches using microbubbles (MBs) and ultrasound to overcome current limitations. The technique provides "stealth" delivery for viral vectors which helps them avoid immune system detection while permitting targeted release within the tumor microenvironment. The UTMD technique holds potential as it enables precise delivery of therapeutic viruses to tumor locations while increasing gene therapy effectiveness.
- Immune Responses and Off-Target Effects
Immune responses
Viral vectors in gene therapy treatments face immune system attacks which generate reactions that lower treatment success rates. Researchers are working to minimize immune reactions by creating viral vectors with reduced immunogenicity while developing methods to control the immune system's response. Microbubbles used in delivery systems protect viral vectors from immune detection which allows for enhanced delivery efficiency. Another approach to minimize immune-related complications involves preconditioning the immune system or administering immunosuppressive drugs.
Off-target effects
Gene-editing tools such as CRISPR-Cas9 create unintended genetic modifications through off-target effects which represent a major risk. Scientists are developing new versions of gene-editing tools which aim to increase precision while decreasing unintended genetic changes. Scientists are creating high-fidelity Cas9 variants and base editors to improve the precision of gene-editing technology. Researchers execute comprehensive preclinical studies with next-generation sequencing (NGS) to detect and reduce unintended mutations. The precision of gene therapy must be improved to reduce unintended effects on non-target cells. Cancer-specific promoters can control therapeutic gene expression so that activation occurs exclusively within cancer cells. Cancer-specific promoters in oncolytic viruses enable selective activity against tumor cells which minimizes unintended off-target effects.
Conclusion
The application of gene therapy in cancer treatment looks highly promising because it allows direct intervention in genetic abnormalities that cause tumors to grow. The development of CRISPR-Cas9 gene-editing technologies alongside sophisticated delivery systems has broadened the scope of gene therapy applications within cancer treatment. Researchers must address several obstacles such as guaranteeing safe and effective gene delivery while reducing immune responses and off-target effects. Upcoming research will concentrate on creating advanced delivery systems that improve precision while lowering immune reactions as well as examining combination therapies that combine different treatment methods for maximum effectiveness along with personalized treatment plans tailored to each tumor's genetic identity. The ongoing development of gene-editing technologies and immune engineering solutions will likely improve gene therapy's precision and effectiveness. The progression of these scientific breakthroughs positions gene therapy to become a pivotal force in cancer treatment which promises better patient outcomes and enhanced quality of life.
References
- Ay, C.; Reinisch, A. Gene therapy: principles, challenges and use in clinical practice. Wien Klin Wochenschr. 2024, 1-11. https://doi.org/10.1007/s00508-024-02368-8.
- Youssef, E.; Fletcher, B.; Palmer, D. Enhancing precision in cancer treatment: the role of gene therapy and immune modulation in oncology. Front. Med. 2025, 11:1527600. https://doi.org/10.3389/fmed.2024.1527600.
- Watanabe, M.; Nishikawaji, Y.; Kawakami, H.; Kosai, K.-i. Adenovirus Biology, Recombinant Adenovirus, and Adenovirus Usage in Gene Therapy. Viruses. 2021, 13, 2502. https://doi.org/10.3390/v13122502.
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells. 2023, 12, 785. https://doi.org/10.3390/cells12050785.
- Soufizadeh, P.; Mansouri, V.; Ahmadbeigi, N. A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights. Lab Anim Res. 2024, 40, 17. https://doi.org/10.1186/s42826-024-00195-6.
- Distributed under Open Access license CC BY 4.0, without modification.
