siRNA-The Silent Guardian of Cellular Health
Definition of RNAi
The natural defense mechanism of RNA interference (RNAi) protects organisms from the entry of alien genes. The RNAi modalities of siRNA and miRNA achieve specific gene expression knockdown through mRNA degradation (siRNA and miRNA) and mRNA translation inhibition (miRNA). The gene silencing capability of siRNA surpasses miRNA because miRNA controls multiple genes concurrently. Pharmaceutical applications utilize siRNA and miRNA to execute different biological functions. RNA interference discovery in 1998 led to siRNA therapeutic development which experienced alternating periods of advancements and challenges. A specific gene expression was silenced by researchers who introduced chemically synthesized siRNA into mammalian cells in 2001 and this resulted in a developmental upsurge. Following early difficulties with stability, specificity and delivery the field of siRNA therapy experienced significant progress through chemical modification and delivery enhancements which revitalized research advancement rapidly in recent years.
siRNA serves as a defensive shield for cells to combat viral infections
- Viral Infections and siRNA Response
siRNA serves as an essential mechanism which protects cells from viral infections. During cellular infection by a virus replication process generates double-stranded RNA (dsRNA). The cell detects this dsRNA as an external invader which activates the RNAi pathway. Dicer processes dsRNA into siRNA fragments which direct the RNA-induced silencing complex (RISC) to destroy viral mRNA and stop viral replication. Researchers have achieved success in using siRNA to degrade mRNA in HIV, hepatitis, and influenza viruses which reduces viral load and prevents infection spread.
- siRNA in Immune Modulation
siRNA functions by targeting viral RNA and engages with the innate immune system. The innate immune system functions as the body's main defense against invading pathogens and siRNA can regulate this defense mechanism. siRNA induces an antiviral state through viral RNA degradation and it may activate the innate immune response upon detection of double-stranded RNA by pattern recognition receptors (PRRs). Type I interferons (IFNs) and various antiviral cytokines emerge as a result of this interaction.
Scientists have the capability to create siRNA molecules which reduce their risk of initiating non-specific immune reactions. Chemical alterations like 2′-fluoro and thioate linkages serve to increase siRNA stability while lessening its immunogenic effects. Bioinformatics methods enable researchers to create siRNAs that bypass immune system activation to ensure therapeutic benefits remain clear of non-specific immune responses.
siRNA in Cellular Stress: A Calming Influence
- Response to Oxidative Stress
siRNA acts as a crucial defense system against oxidative stress which plays a substantial role in causing diseases such as cancer along with neurodegenerative and cardiovascular conditions. When cells produce reactive oxygen species faster than they can detoxify them they experience oxidative stress. Sufficient detoxification of reactive oxygen species is crucial because inadequate processing leads to cellular damage and activation of stress response pathways.
Researchers can utilize siRNA to target genes linked to stress which results in modulation of the cellular reaction to oxidative stress conditions. Research demonstrates that siRNA which targets the IRE1α gene diminishes apoptosis caused by ER stress in cells that face high glucose concentrations. Diabetic peripheral neuropathy (DPN) demonstrates particular relevance due to the neuronal damage caused by persistent endoplasmic reticulum stress. siRNA targeting IRE1α reduces CHOP and Caspase-12 gene expression which protects cells against cell death triggered by oxidative stress.
- siRNA in Apoptosis Regulation
siRNA serves as a critical regulator of apoptosis which represents the programmed mechanism of cellular death. The programmed cell death mechanism known as apoptosis exists as a strictly controlled process vital for tissue balance and removal of compromised cells. Dysregulated apoptosis plays a role in the development of multiple diseases including cancer together with neurodegenerative disorders.
siRNA acts as a regulator in apoptosis pathways by interfering with essential regulatory genes. Research demonstrates that siRNA directed against the IRE1α gene prevents activation of the JNK pathway which plays a part in stress-induced apoptosis. The use of siRNA enables manipulation of the intrinsic apoptosis pathway by targeting Bax and Bcl-2 genes which control the equilibrium between pro-apoptotic and anti-apoptotic signals.
siRNA in Cellular Metabolism: A Fine-Tuning Mechanism
siRNA now represents an effective approach to control cellular metabolism through the targeting of essential metabolic genes which results in energy homeostasis modulation. Researchers found that siRNA aimed at the PGC-1α gene affects mitochondrial biogenesis and oxidative phosphorylation which are essential for energy production. PGC-1α siRNA treatment in cancer cells demonstrates inhibitory effects on metastasis through the reversal of the Warburg effect which fuels rapid cell division. Targeted methodologies enable precise metabolic pathway regulation which shows promise as a therapeutic option for metabolic diseases.
siRNA demonstrates powerful effects on lipid and glucose metabolism regulation with documented success in treating metabolic disorders. Using siRNA to target the AT1R gene reduced lipid accumulation in HepG2 cells by increasing PPARα expression and activating its downstream genes. This biological mechanism serves as a key regulator of lipid metabolism while providing protection against non-alcoholic fatty liver disease (NAFLD). SiRNA targeting SIRT3 manages mitochondrial operations and sustains energy balance by deacetylating key enzymes involved in the TCA cycle and fatty acid decomposition. Controlling glucose and lipid metabolism remains essential while siRNA presents a promising therapeutic approach for metabolic diseases such as diabetes and obesity.
siRNA for Cellular Health: Role in Tumor Treatment
SiRNA demonstrates multiple benefits in cancer treatment because it provides precise targeting capabilities along with versatile applications and the ability to reach genes that traditional drugs cannot affect. Researchers can develop siRNA molecules to silence multiple cancer-associated genes responsible for cell cycle regulation, proliferation, metastasis, angiogenesis, apoptosis, DNA repair processes, immune system evasion, metabolic control, and cancer development. The extensive targeting potential of siRNA positions it as a strong option for tackling cancer's intricate genetic nature.
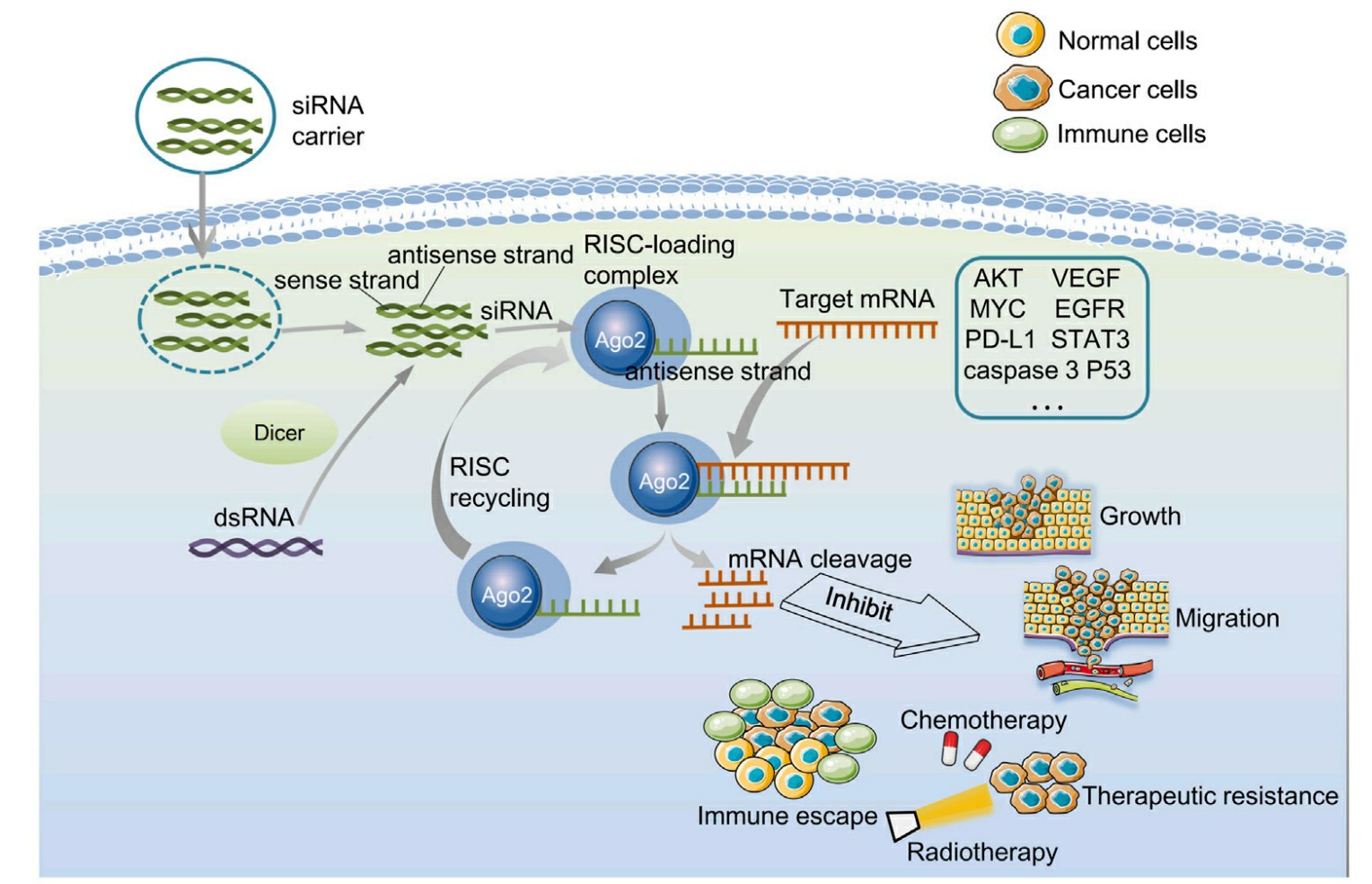 Fig 1. Biological characterization of gene silenced by endogenous siRNA and exogenous siRNA1,6.
Fig 1. Biological characterization of gene silenced by endogenous siRNA and exogenous siRNA1,6.
- Mechanisms and Targets
The RNAi mechanism enables siRNA to function as an effective cancer treatment tool by silencing genes that drive cancer growth. A wide range of molecular pathways can be targeted by siRNA which includes cell cycle regulation along with proliferation, metastasis, angiogenesis and apoptosis as well as DNA damage repair immune evasion metabolism and oncogenes. siRNA directed against the M2 subunit of ribonucleotide reductase (RRM2) demonstrates potential to block metastasis through Warburg effect reversal which affects metabolic programming in fast-growing cells. siRNA directed at PKN3 has proven effective for treating pancreatic cancer that has progressed to advanced stages or metastasis.
- Preclinical Applications and Examples
The production of 21- and 22-nucleotide siRNA through ribonuclease III enzyme activity on dsRNA proved to have gene silencing capabilities in research experiments. Researchers established that siRNA could inhibit genetic activity across multiple mammalian cell lines such as HeLa and human HEK. The demonstration revealed that siRNA functions can be transformed into a new class of unconventional medication designed to target and suppress genes that cause or promote diseases. Gene silencing via siRNA becomes essential when disease targets remain inaccessible to small molecules and antibodies or proteins. Both in-vivo and in-vitro studies demonstrated significant suppression of abnormal cancerous cell proliferation through siRNA-mediated silencing. siRNA demonstrates significant potential as a chemosensitizing agent which makes drug-resistant cancer cells more susceptible to chemotherapy treatments. Researchers used siRNA against SOX18 as a breast cancer treatment strategy in their experiment. siRNA suppresses SOX18 expression both at mRNA and protein levels in breast cancer cells. When researchers silence SOX18 expression they observe apoptotic effects in breast cancer cells and achieve a marked decrease in both tumor growth and metastasis. The application of siRNA to lower SOX18 expression leads to a significant decrease in PDGFB, RhoA, IGF-1R, and MMP-7 expression which helps to impede breast cancer cell progression.
- Challenges
The potential of siRNA therapy remains limited because siRNA molecules are unstable and quickly cleared from the bloodstream while requiring advanced delivery systems. Scientists have devised numerous chemical modifications and delivery systems to overcome these obstacles. Researchers have utilized lipid nanoparticles (LNPs) to shield siRNA from degradation while boosting its cellular uptake. Researchers have explored bioresponsive materials that release siRNA upon encountering specific cellular conditions to enhance both the precision and efficacy of siRNA delivery systems.
The Future of siRNA for Cellular Health
- Enhanced endosomal escape
siRNA within endosomes maintains identical characteristics to its extracellular counterpart. siRNA requires endosomal disruption to achieve therapeutic effects in the cytosol. siRNA molecules face a high likelihood of degradation at lysosomal pH 4.5 when they remain trapped inside the endosome. The main barrier to siRNA therapeutic use remains endosomal escape. Advancements in stimulus-responsive polymers are necessary in the current context to enable drug release upon exposure. A research team created an ultrasound-responsive polymersome constructed from PEO-b-poly(DEA-stat-MEMA) block copolymer to determine its drug delivery capabilities inside cancer cells and its overall antitumor effectiveness in living organisms. The polymersome demonstrated effective endosomal escape capabilities. A new study explored sulfonated PEIs that were covalently attached to pyropheophorbide-α for photoactivation and modified with amines to achieve controlled endosomal escape called sulfo-pyro-PEI. Study results demonstrated that the siRNA emitted from the system when photostimulated. Researchers developed multivalent peptide-functionalized bioreducible polymers to enhance endosomal escape mechanisms. Research demonstrates that achieving the right amount of hydrophobic side chains is necessary for micelle formation and cellular uptake yet excessive hydrophobic chains can reduce endosomal escape. Future research must focus on structural optimization of polymers and nanoparticle design to resolve the endosomal escape challenge.
- Conjugation with proteins and antibodies
Effective siRNA activity requires successful endosomal escape. By conjugating siRNA with antibodies and proteins you can increase its serum half-life which leads to enhanced potency. The IgE represents one of the antibodies that plasma cells produce. After IgE synthesis it persists in blood and tissue fluids for several weeks. It is believed the IgE-siRNA complex maintains equivalent longevity in circulation. The tested siRNA molecule demonstrated reduced potency compared to its unconjugated counterpart. The use of albumin as a conjugation partner can assist in delivering siRNA to cancer cells and improve its pharmacokinetic properties. The lower effectiveness of the IgE-siRNA complex raises doubts about whether future research will produce equivalent results. We must continue exploring additional alternatives until we can significantly boost siRNA effectiveness and its pharmacokinetic profiles.
- Tissue targeting and cellular uptake
Successful cancer therapy faces the challenge of delivering treatment to unintended locations. Nanoparticle encapsulation of siRNA protects it from nucleases and body clearance while enabling targeted release when combined with specific targeting ligands. Nanoparticles with entrapped siRNA measuring under 150 nm successfully reach hepatocytes yet similar fenestrations do not exist in other tissues which limits entry. Galactose-linked liposomes represent targeted nanocarriers which scientists confirmed as effective in increasing drug effectiveness at liver locations. Using PEG conjugation provides an alternative way to prevent macrophages from identifying drug delivery systems. The use of tumor targeting through PEGylation combined with passive diffusion via EPR proves insufficient for targeting all cancer types. Facilitated or active diffusion stands out as the superior delivery method to minimize off-site siRNA accumulation and increase intracellular delivery into cancer cells. The delivery of siRNA through ligand-conjugated endocytosis to target cells will prevent side effects while simultaneously boosting siRNA effectiveness. In the future research must explore facilitated and active transportation techniques together with external stimulus mediation methods such as magnetic field applications and ultrasonic waves alongside the use of laser lights and sound and light. Researchers need to conduct additional studies on the system of on-demand siRNA release.
- Multifunctional approach
Multiple obstacles stand in the way of realizing the full therapeutic potential of siRNA. Challenges to siRNA therapy include endosomal escape issues, low cellular uptake rates, fast excretion processes, degradation by nucleases and immune stimulation. The creation of an ideal carrier demands difficult work when combining all solutions within one delivery system. A versatile system that combines most attributes of the perfect delivery mechanism has the potential to substitute current solutions. The system performance improves when endosomal escape motifs are added to a multifunctional platform without affecting cellular uptake or targeting ligand potency. By adding the targeting ligand the system reduces non-specific accumulation while enhancing site-specific delivery. RNA stability against nuclease degradation can be achieved through chemical alteration at the 2′ position while cellular uptake can be improved by lipid fusion. The integration of every motif into a single system would lead to greater complexity in the delivery apparatus. The comprehensive examination of the physiochemical properties of each component is crucial because they need to enhance each other's function while operating independently.
References
- Teng, Xin-Qi, et al. "Small interfering RNA for gliomas treatment: overcoming hurdles in delivery." Frontiers in Cell and Developmental Biology 10 (2022): 824299. https://doi.org/10.3389/fcell.2022.824299.
- Levanova, Alesia, and Minna M. Poranen. "RNA interference as a prospective tool for the control of human viral infections." Frontiers in Microbiology 9 (2018): 2151. https://doi.org/10.3389/fmicb.2018.02151.
- Deng, Kaili, Dongxue Yang, and Yuping Zhou. "Nanotechnology-based siRNA delivery systems to overcome tumor immune evasion in cancer immunotherapy." Pharmaceutics 14.7 (2022): 1344. https://doi.org/10.3390/pharmaceutics14071344.
- Wang, Min, et al. "Neutrophil-like cell membrane-coated metal-organic frameworks for siRNA delivery targeting NOX4 to alleviate oxidative stress in acute ischemic injury." Acta Biomaterialia 196 (2025): 487-505. https://doi.org/10.1016/j.actbio.2025.02.061.
- Kalita, Tutu, et al. "siRNA functionalized lipid nanoparticles (LNPs) in management of diseases." Pharmaceutics 14.11 (2022): 2520. https://doi.org/10.3390/pharmaceutics14112520.
- Distributed under Open Access license CC BY 4.0, without modification.
