Adeno-Associated Virus (AAV) Vectors in Gene Therapy for Cystic Fibrosis
Overview of Cystic Fibrosis as a Genetic Disorder
The condition known as cystic fibrosis (CF) represents a prevalent autosomal-recessive genetic disease resulting from mutations in one gene which produces the cystic fibrosis transmembrane conductance regulator (CFTR) protein. The CFTR protein functions as an anion channel within the apical membrane of epithelial cells and enables chloride and bicarbonate ions to cross the cellular membrane. The CFTR gene mutation causes cystic fibrosis which impacts at least 70,000 people worldwide and has been found to have almost 2000 sequence variations. The ΔF508CFTR mutation represents the most prevalent CFTR alteration which emerges from the deletion of a nucleotide triplet that eliminates a phenylalanine at position 508. Around 70% of CF patients have two ΔF508 mutation copies while 90% possess one copy. Mutations in the CFTR gene lead to various organ dysfunctions such as serious lung infections and pancreatic failure along with intestinal obstruction and male infertility plus nutritional deficits. CF organ disease commonly features thick secretions together with low pH levels resulting from defective bicarbonate transport.
What is the Gene Therapy
Gene therapy represents an advanced medical technique which seeks to manage or prevent health conditions through the repair, replacement, or modification of human genes. The field of innovative medicine takes a direct approach to treat genetic disorders by targeting and fixing the fundamental genetic defects that cause them. Traditional treatments target disease symptoms but gene therapy offers sustainable or permanent cures by fixing genetic mutations responsible for the condition. Gene therapy works by delivering genetic material directly into the cells of the patient. Different delivery techniques enable gene therapy including viral vectors alongside non-viral vectors and other systems. Therapeutic genetic material exists in multiple formats including functional gene copies to replace defective ones, RNA molecules that adjust gene expression levels, and precise gene-editing tools like CRISPR/Cas9 which target specific mutations for correction.
The Potential of Gene Therapy to Address Cystic Fibrosis
The potential of gene therapy to revolutionize CF treatment lies in its ability to correct the fundamental genetic defect that leads to the disease. Traditional treatments for cystic fibrosis target symptom management and disease complications through antibiotics for lung infections and mucolytics for mucus reduction while enzyme replacement therapy treats digestive problems. These therapies only offer temporary quality of life improvements for patients because they cannot fix the genetic defect and typically do not change the disease's long-term progression. Gene therapy presents a more direct cure-oriented solution by delivering a working CFTR gene to targeted airway epithelium cells. Gene therapy can restore CFTR protein functionality which would repair ion transport issues while normalizing mucus production and decreasing lung infection occurrences and severity. Patients who receive this treatment will experience improved respiratory function which will result in beneficial changes to their overall health by reducing digestive issues and improving life quality.
Types of vector in gene therapy for cystic fibrosis
- Viral Vectors
-
Lentiviral vectors
Lentiviral vectors stand out as top choices for cystic fibrosis (CF) gene therapy because they efficiently deliver genes to airway epithelial cells and integrate those therapeutic genes into the host genome. Animal model studies show that these vectors restore CFTR function effectively in both rats and pigs. Experimental research demonstrates that lentiviral vectors restore CFTR functionality in CF mice and rat nasal epithelium. Researchers have demonstrated that lentiviral vectors can effectively transduce human CF airway epithelial cells cultured in vitro leading to enhanced CFTR activity. Lentiviral vectors demonstrate efficient transduction capabilities in cell populations regardless of their division status. The therapeutic gene becomes stably expressed over a long duration once it integrates into the host genome. -
Adeno-associated virus (AAV) vectors
AAV vectors stand out as another viable candidate for treating cystic fibrosis through gene therapy. These vectors achieve long-lasting gene expression through mechanisms that do not involve integration into the host genome and exhibit minimal immune response. Preclinical studies indicate that AAV vectors demonstrate potential for delivering the CFTR gene to the cells lining the airways. Due to the minimal immune responses they trigger AAV vectors can be used multiple times in treatments. -
Airway transduction vectors derived from respiratory viruses
Even though lungs have defense systems against external pathogens, respiratory viruses manage to bypass these defenses which enables them to establish infections and multiply. Paramyxovirus-derived recombinant vectors like human parainfluenza virus (PIV), respiratory syncytial virus (RSV), and murine SeV demonstrate efficient transduction capabilities for both in vitro airway epithelium and mouse lung tissue in vivo. The vectors produced from negative-sense, single-stranded RNA viruses demonstrated only temporary expression and generated powerful adaptive immune responses during transduction. Their clinical application remains restricted because of these disadvantages combined with challenges in vector production. Human respiratory disease DNA viruses consist of nonenveloped double-stranded DNA (dsDNA) viruses from the Adenoviridae family and the newly identified single-stranded DNA virus HBoV1 from the Parvoviridae family. Acute respiratory tract infections in young children are caused by HBoV1. Children have a 13% seroprevalence of HBoV1 capsid-specific immunoglobulin G whereas adults show a 59% seroprevalence. Seroconversion fails to stop repeated infections with HBoV1. Researchers have successfully cloned the complete genome sequence of HBoV1. Transfection of the cloned HBoV1 duplex genome into HEK293 cells generates infectious HBoV1 virions despite these cells not supporting natural HBoV1 infection. The polarized human HAE cells demonstrate efficient infection by HBoV1 in vitro which leads to progeny production. -
Non-viral Vectors
Research into CF gene therapy includes examinations of non-viral vectors such as liposomes and nanoparticles. These vectors present multiple benefits that include minimal immunogenic response and increased genetic payload capacity. Scientists can create nanoparticles that encapsulate therapeutic genes so they deliver these genes to target cells while generating little to no immune response. Non-viral vectors produce lower immune responses which leads to fewer adverse effects. Non-viral vectors possess the capability to transport more genetic material than viral vectors. Targeting ligands can be attached to these vectors to increase their specificity towards airway epithelial cells.
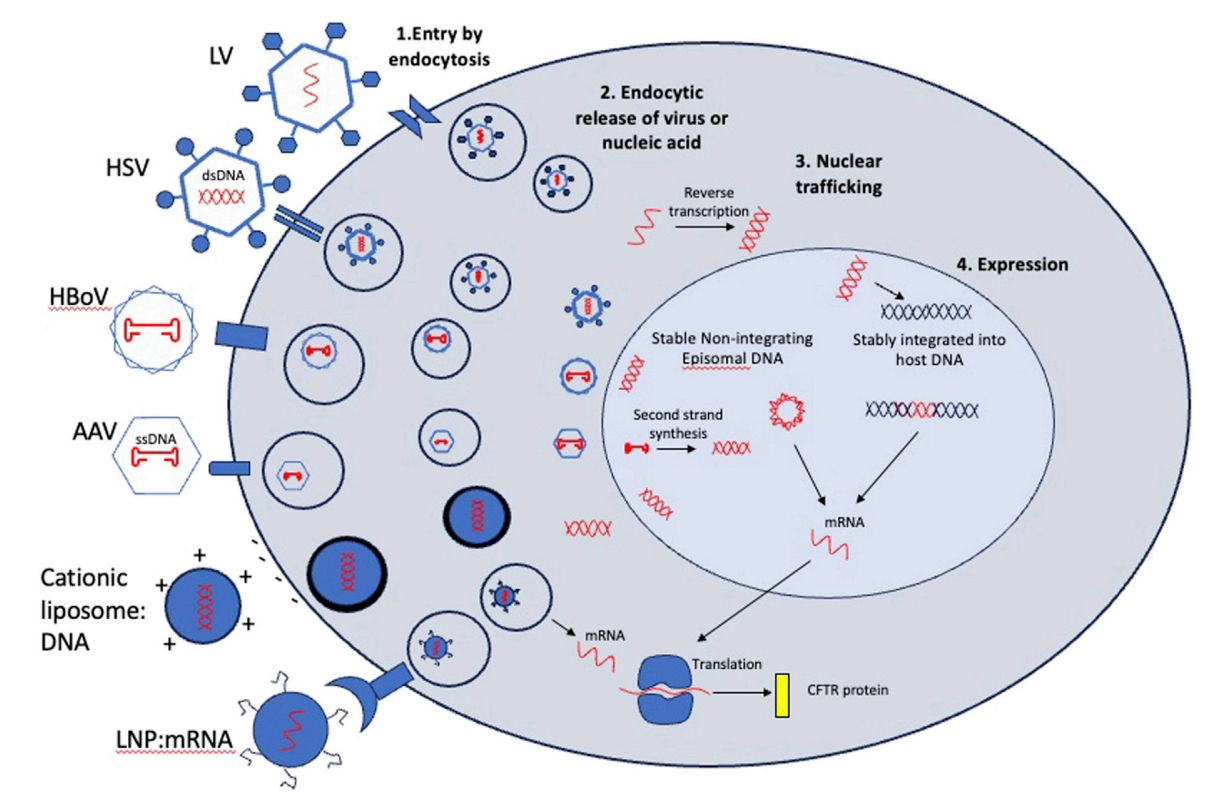 Fig. 1 Entry mechanisms and post-entry processing of viral and non-viral vectors considered for CFTR gene therapy1,5.
Fig. 1 Entry mechanisms and post-entry processing of viral and non-viral vectors considered for CFTR gene therapy1,5.
What is the Best Vector in Gene Therapy for Cystic Fibrosis
AAV vectors represent the preferred choice for CF gene therapy because they exhibit minimal immune response and sustain gene expression over extended periods. Recent research has pinpointed particular AAV serotypes that show strong targeting ability towards pulmonary epithelial cells which are CF's main target cells. Research proved that AAV2 can infect polarized airway epithelia. The Phase IIb clinical study showed transient gene transfer because the vector genome amounts dropped from 0.6 copies per brushed airway cell on day 14 to only 0.1 copies by day 30. No vector was detected at 90 days. The measurement of vector genome instead of transgene expression in these studies likely resulted in an overestimation of transduction because post-entry barriers in virion processing following infection restrict both nuclear translocation and mRNA expression. The patient tolerated AAV2-CFTR delivery safely and well but this treatment did not achieve significant functional efficacy or sustained disease modification. Scientists discovered new naturally occurring AAVs that demonstrate enhanced targeting abilities for different airway cell populations. AAV5 demonstrated a 10-fold increase in transduction efficiency compared to AAV2 within non-human primate lung tissues. Recent research programs utilize novel AAV vectors which have evolved capsids through mutagenesis or recombination and selected for enhanced targeting of airway cells.
The Reason for Choosing the Best Vector in CF Gene Therapy
AAVs remain popular for in vivo gene delivery due to their low immunogenicity and episomal expression of genetic material which avoids integration into host chromosomes. AAV6 achieves up to 80% transduction efficiency in airway epithelial cells within mice models and displays reduced immunogenicity when compared to AAV2. The AAV6.2 variant represents a further modification that has shown greater transduction efficiency in airway epithelial cells of both mice and humans. The ability of AAV6 to penetrate into the thick mucus layer of CF patients represents a crucial benefit. The novel AAV capsid demonstrated successful CFTR function restoration across both laboratory and living organism models. AAV204 demonstrates superior transduction efficiency in bronchial and nasal epithelial cells within pulmonary regions of CF patients relative to AAV6.
Current Barriers and Recent Developments
- Challenges in Delivering AAV to Target Tissues and the Impact of Administration Route
The delivery of AAV vectors to specific tissues represents a considerable obstacle in gene therapy treatments for CF. The effectiveness of AAV vector delivery depends on various aspects such as the chosen administration route alongside the specific AAV serotype used and any physical barriers found within the target tissue. In CF gene therapy the main target is the airway epithelium however delivering genes effectively is difficult due to the thick mucus layer and fast epithelial cell turnover. The direct targeting of the airway epithelium in CF treatments has included the exploration of intranasal and nebulized AAV vector delivery methods. These delivery routes increase local transduction efficiency but also heighten immune system exposure which could activate immune responses. The success of AAV-based gene therapy for cystic fibrosis depends heavily on finding an administration route that optimizes both transduction efficiency and immune response management.
- Immunogenicity of AAV and Strategies to Mitigate Immune Responses
The immune response generated by AAV vectors stands as a significant obstacle in achieving clinical success for gene therapy treatments. The therapy's efficacy and safety face limitations because AAV vectors trigger both innate and adaptive immune responses. Pattern recognition receptors including Toll-like receptors (TLRs) initiate the innate immune response through the detection of viral components. During the adaptive immune response against AAV vectors neutralizing antibodies and cytotoxic T cells are generated to target the AAV capsid. Multiple approaches have been developed to decrease immune responses to AAV vectors. Scientists modify the AAV genome to lessen innate immune pathway activation. Removing CpG motifs from the AAV genome lowers TLR9 activation while increasing transgene expression. The immune response can be further diminished by integrating TLR9-inhibitory sequences into the AAV genome.
- Recent Advancements in AAV Vector Technology
The latest developments in AAV vector technology aim to increase transduction efficiency while simultaneously lowering immunogenicity and boosting gene therapy safety. The engineering of AAV capsids to improve tissue specificity while lowering vector dosage represents a major breakthrough in vector technology. The AAV6.2 variant demonstrates better transduction efficiency in airway epithelial cells which positions it as a strong candidate for CF gene therapy. Dual AAV vectors represent a recent advancement because they divide large transgenes between two vectors to deliver genetic material. The dual AAV vector system solves the typical payload capacity restrictions of traditional AAV vectors allowing the delivery of bigger therapeutic genes. Self-complementary AAV vectors improve transduction efficiency because they supply a double-stranded DNA template that supports transcription.
Preclinical Studies using AAV Vectors for CF Gene Therapy
- Safety and Efficacy of AAV2-CFTR Vectors
The development of AAV-based vectors for CF gene therapy has been greatly driven by preclinical studies demonstrating their safety and effectiveness. Researchers discovered that primary nasal polyp cell cultures from CF patients could be effectively transduced with the CFTR gene using recombinant AAV2 (rAAV2) vectors in early studies. Researchers found that CFTR RNA and protein remained detectable within rabbit bronchi for a period that extended up to 6 months post-administration. In Rhesus macaques vector-specific DNA and RNA expression continued for 180 days. The studies presented no signs of inflammatory or toxic responses which supports the safe use of AAV2 vectors in CF gene therapy applications.
- Repeated Dosing and Immune Responses
CF gene therapy faces the challenge of requiring multiple doses because the airway epithelial cells naturally renew themselves. The repeated delivery of AAV2-CFTR vectors proved possible in New Zealand white rabbits and Rhesus monkeys even though neutralizing antibodies formed. As antibody levels increased researchers found sustained transgene expression which demonstrated that multiple AAV vector doses did not compromise safety. The reduction in vector transduction magnitude from repeated dosing underscores the requirement for developing methods to minimize immune responses.
- Preclinical Models and Outcomes
Studies using animal models such as pigs, sheep, ferrets, and mice have provided essential data to assess AAV vector effectiveness for CF gene therapy. Pigs were used to test AAV vectors with high airway epithelial tropism which demonstrated efficient CFTR expression and anion transport restoration. Research findings indicate that AAV vectors deliver normal CFTR function restoration and CF phenotype improvement when used in animal models. New AAV capsids like AAV204 have shown better pulmonary cell targeting and increased transduction effectiveness in CF patient treatments.
- Future Directions of Gene Therapy for Cystic Fibrosis
The transfer of AAV gene therapy for CF into clinical use encounters multiple obstacles such as maintaining vector stability and appropriate storage conditions along with scalable manufacturing and immunogenicity concerns. The need for ultra-low temperature storage conditions for AAV vectors creates logistical challenges because it maintains their stability. The expensive and intricate nature of manufacturing processes restricts the broad distribution of medical products. Immunogenicity poses a significant challenge because both pre-existing and induced immune responses can decrease therapy effectiveness during repeated treatments. The successful clinical application of AAV-based gene therapies for CF requires the resolution of existing challenges. Ongoing research seeks to improve AAV vector design by increasing transduction efficiency while minimizing immunogenic responses and expanding payload capacity through capsid engineering along with dual AAV and self-complementary AAV vectors. The production of safe and consistent vectors requires advancements in manufacturing processes through scalable production methods and robust quality control. Personalized medical strategies which include individual CFTR mutation-based treatment alongside combination therapies with CFTR modulators or anti-inflammatory medications show potential for better therapeutic results. Advancements in these fields remain essential to remove existing obstacles and achieve the complete potential of AAV gene therapy for cystic fibrosis.
References
- Plasschaert, L.W.; MacDonald, K.D.; Moffit, J.S. Current landscape of cystic fibrosis gene therapy. Front. Pharmacol. 2024, 15:1476331. https://doi.org/10.3389/fphar.2024.1476331.
- Cooney, A.L.; McCray, P.B., Jr.; Sinn, P.L. Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward. Genes. 2018, 9, 538. https://doi.org/10.3390/genes9110538.
- McCarron, A.; Ling, KM.; Montgomery, S.T. Lentiviral vector gene therapy and CFTR modulators show comparable effectiveness in cystic fibrosis rat airway models. Gene Ther. 2024, 31, 553-559. https://doi.org/10.1038/s41434-024-00480-y.
- Sui, H.; Xu, X.; Su, Y.; Gong, Z.; Yao, M.; Liu, X.; Zhang, T.; Jiang, Z.; Bai, T.; Wang, J.; Zhang, J.; Xu, C.; Luo, M. Gene therapy for cystic fibrosis: Challenges and prospects. Front. Pharmacol. 2022, 13:1015926. https://doi.org/10.3389/fphar.2022.1015926.
- Distributed under Open Access license CC BY 4.0, without modification.
