The Cardiac Silent Treatment-siRNA's Rhythm Reset
Introduction of siRNA in Cardiac Diseases
Cardiovascular diseases (CVD) typically develop gradually over time and remain chronic conditions. The therapy of CVD has achieved considerable progress but current drugs show many negative characteristics including toxicity and resistance. The natural cellular defense mechanism of siRNA provides superior benefits compared to pharmacological treatments because it effectively targets CVD-specific pathogenic genes with high potency while maintaining low toxicity. The use of siRNA extends to hyperlipemia, ischemia-reperfusion and choroidal neovascularization models and also helps identify critical genes associated with CVD. siRNA uses the natural RNA interference (RNAi) pathway to target and break down mRNA molecules which results in decreased production of specific disease-related proteins. A specific approach demonstrated potential results in treating various cardiovascular conditions such as atherosclerosis along with heart failure and hypertension.
Introduction: The Heart's Electrical Storm
CVD cover multiple conditions affecting the heart and blood vessels which remain the top cause of global mortality and present major health hurdles. This category covers cardiac issues including myocardial infarction (MI) and stroke along with heart failure and arrhythmias as well as various vascular diseases such as atherosclerosis hypertension peripheral artery disease valvular heart disease and congenital heart disease. Cardiovascular diseases resulted in approximately 20.5 million deaths globally in 2021. When cells receive double-stranded RNA molecules either through direct cytoplasmic introduction or from external environmental uptake they process these molecules into siRNAs. The enzyme Dicer enters the cell and cuts long dsRNA molecules into shorter segments called siRNAs. The guide strand of siRNA leads the RISC complex to specific mRNA targets which results in its cleavage or degradation and blocks protein synthesis.
siRNA function: The Heart's Molecular Mute Button
- Therapeutic potential: Precision silencing of faulty cardiac ion channels
siRNA shows promise for treating cardiac conditions because it targets malfunctioning cardiac ion channels for silencing. Ion channel dysfunction usually triggers the development of arrhythmias such as atrial fibrillation and ventricular tachycardia. Scientists can develop siRNA molecules that attach to the mRNA of these ion channels which leads to decreased channel expression and may restore normal cardiac electrical function. The use of siRNA to target the mRNA of particular ion channels responsible for arrhythmogenesis shows encouraging results in preclinical studies by showing potential to decrease both arrhythmia occurrence and intensity.
- Advantages over traditional antiarrhythmics
In comparison with other conventional drugs, siRNA have many advantages: Target site selection becomes easier and more flexible due to the sequence-specific nature of target mRNA and the complementary properties of siRNA. The inhibitory effects of siRNA can be directed at various sections of a single mRNA molecule. The precise targeting by siRNA towards the cognate transcript occurs through its sufficient length and strong homology to the target region. Cells contain siRNA that remains inactive if they lack appropriate targets. The most attractive feature of RNAi in antiviral applications is its ability to achieve exclusive specificity without any adverse side effects. siRNA exhibits long-term biological effects after being delivered through viral or plasmid vectors.
siRNA's Precision Strike: Targeting Arrhythmia Genes
- The use of KCNH2 siRNA to silence the "hERG" Channel
Long QT syndrome (LQTS) represents a genetic condition that extends QT intervals in electrocardiogram readings which results in heightened arrhythmia risk and sudden cardiac death. The hERG potassium channel encoded by KCNH2 gene mutations causes the LQT2 subtype of LQTS. Researchers found that siRNA targeting KCNH2 shows effectiveness in silencing the hERG channel to prevent Long QT Syndrome. Researchers have shown that using allele-specific RNAi to target mutated KCNH2 mRNA can restore normal action potential durations and potassium currents in cardiomyocytes from patients with diseased phenotypes.
- Targeting SCN5A to Reset Sodium Channels
Brugada syndrome (BrS) is a genetic condition that significantly increases patients' risk of sudden cardiac death through ventricular fibrillation. Mutations in the cardiac sodium channel encoding gene SCN5A stand as the primary cause of Brugada syndrome. The targeted silencing of SCN5A mRNA through siRNA treatment can restore proper sodium channel function and prevent deadly arrhythmias in Brugada syndrome patients.
- RyR2 siRNA therapy
Catecholaminergic polymorphic ventricular tachycardia (CPVT) stands as a genetic disease which triggers arrhythmias when people engage in physical activity and may lead to unexpected cardiac death. Mutations in the RYR2 gene produce the ryanodine receptor 2 protein which leads to the CPVT disorder. CPVT patients showed decreased calcium leakages and arrhythmic events when they received siRNA targeting the RYR2 gene. The specific silencing of the mutated RYR2 mRNA through siRNA treatment restores normal calcium handling and blocks ventricular tachycardia development.
siRNA Delivery: Hitting the Heart's Bullseye
Challenges: Avoiding Off-Target Effects, Penetrating Cardiac Tissue
Multiple substantial obstacles exist in transporting siRNA to the heart. The main challenge is preventing off-target effects where siRNA might silence unintended genes causing unexpected results. The dense extracellular matrix of cardiac tissue along with the hydrophobic nature of cell membranes makes tissue penetration difficult. SiRNA molecules remain unable to diffuse through the plasma membrane's lipid bilayer because of their highly anionic nature and hydrophilic properties. The body's reticuloendothelial system rapidly eliminates siRNA from the bloodstream which triggers immune activation.
Breakthrough Strategies
- LNPs (Lipid Nanoparticles): Intravenous Delivery to Cardiomyocytes
LNPs present a potential delivery method for siRNA molecules. The lipid bilayer in LNPs acts as protective packaging around siRNA which increases its stability and provides defense against degradation. When ligands are attached to nanoparticles their targeting specificity towards cells increases and their uptake by cardiomyocytes becomes more efficient. Researchers use LNPs to deliver siRNA against the TGF-β1 gene to heart tissue damage sites for cardiac repair stimulation. Research indicates this method demonstrates strong potential for decreasing fibrosis while enhancing cardiac function.
- Cardiac-Targeted Peptides: Homing to Injured or Fibrotic Areas
Researchers have developed a new method where cardiac-targeted peptides serve as delivery vehicles for siRNA. Targeted delivery of these peptides is achieved through their specific binding to receptors that are overexpressed in cardiac tissue that has sustained injury or developed fibrosis. Researchers have used peptides obtained from fibroblast activation protein (FAP) as carriers to transport siRNA to damaged heart tissue where they have achieved successful gene silencing and tissue restoration. The targeted delivery method reduces unwanted effects while improving siRNA-based treatment success.
- Electroporation: Temporary Pores for Direct siRNA Uptake
Electroporation uses electrical pulses to create temporary cell membrane pores which enable siRNA uptake. Researchers have utilized this approach to deliver siRNA directly to cardiac tissue while maintaining both high efficiency and specificity. The technique of electroporation which produces temporary cell membrane pores enables more efficient siRNA entry into cardiomyocytes and thereby improves therapeutic effectiveness.
siRNA in Cardiac preclinical study
- Inclisiran in preclinical study
Inclisiran became the first siRNA drug to reduce liver production of proprotein convertase subtilisin/kexin type 9 (PCSK9) following joint approval from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). PCSK9 is an enzyme generated by liver cells which manages low-density lipoprotein receptor metabolism and results in higher LDL cholesterol levels that elevate the risk of atherosclerotic cardiovascular disease development. The GalNAc "trident" connects the two modified RNA strands to allow inclisiran immediate uptake by hepatic ASGPR following subcutaneous administration. After entering hepatocytes through endosomal pathways inclisiran attaches to RISC which triggers the degradation of PCSK9 mRNA thereby blocking PCSK9 synthesis and secretion. Non-human primate preclinical studies showed that inclisiran reduced plasma PCSK9 levels by over 80% and serum LDL-C levels by 60% with effects persistent for over 30 days. The success of this therapy has opened new research opportunities for siRNA treatments targeting various therapeutic areas beyond cardiovascular diseases.
- Olpasiran in preclinical study
Olpasiran represents the initial siRNA therapy specifically engineered to decrease apolipoprotein(a) mRNA levels within liver cells targeting Lipoprotein(Lp)(a) which causes both atherosclerosis and cardiac valvular harm. Observational and preclinical research demonstrates that siRNA can lower Lp(a) levels which reduces the risk of multiple cardiovascular diseases including coronary and peripheral atherosclerosis as well as heart failure and valvular disease.
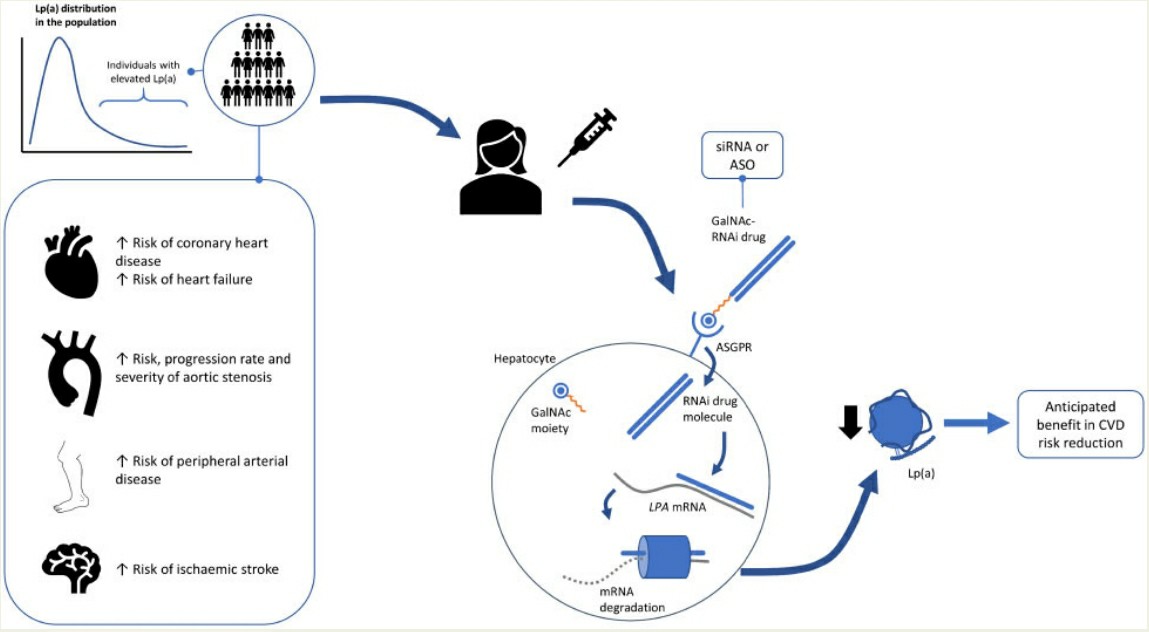 Fig. 1 Mechanism of action of Lp(a)-lowering siRNA therapy, and potential clinical benefits1,6.
Fig. 1 Mechanism of action of Lp(a)-lowering siRNA therapy, and potential clinical benefits1,6.
- siRNA Targeting GJA5 (Connexin-40) in Preclinical Models
Recent studies have targeted GJA5 using siRNA because it encodes connexin-40 and is associated with atrial fibrillation which stands as the most common cardiac arrhythmia. The gap junction protein connexin-40 performs an essential function in the electrical coupling between cardiomyocytes. The research team intends to use siRNA to silence the GJA5 gene and thereby alter cardiac electrical activity to lower AFib occurrences. The application of siRNA to reduce GJA5 expression in preclinical models has led to positive effects on cardiac electrophysiological properties.
- TNF-α siRNA
Arrhythmias emerge as the leading cause of both morbidity and mortality in patients who have suffered a myocardial infarction. The development of arrhythmias depends largely on inflammation because TNF-α acts as a central inflammatory cytokine in this process. Scientists are examining TNF-α siRNA as a treatment method to inhibit TNF-α expression which helps reduce inflammation and protects cardiomyocytes from damage. Animal models of MI used in preclinical research show that TNF-α siRNA substantially lowers TNF-α concentrations while simultaneously reducing inflammation and enhancing heart function.
The Future: A Silent Heart's New Song
The potential of siRNA therapy for cardiac applications looks promising because next-generation siRNA technology advancements combined with AI-assisted design lead to better treatment options. Researchers are developing next-generation siRNA therapies with improved cardiac tropism to ensure that the siRNA molecules reach heart tissue exclusively which reduces off-target effects and boosts therapeutic effectiveness. Research focuses on developing intelligent release systems that control and sustain siRNA delivery to maximize its therapeutic benefits. AI-assisted design represents another groundbreaking development within this research domain. Researchers achieve enhanced precision in identifying arrhythmia targets through the application of artificial intelligence technologies. This technology reveals the key molecular pathways and genes responsible for arrhythmias like AFib and VT which enables researchers to design siRNA molecules capable of effectively silencing those specific targets. Through this approach drug development speeds up while simultaneously heightening the probability of achieving positive clinical results.
References
- Swerdlow, Daniel I., et al. "Treatment and prevention of lipoprotein (a)-mediated cardiovascular disease: the emerging potential of RNA interference therapeutics." Cardiovascular Research 118.5 (2022): 1218-1231. https://doi.org/10.1093/cvr/cvab100.
- Tong, Liangnan, et al. "Research status and prospect of non-viral vectors based on siRNA: a review." International journal of molecular sciences 24.4 (2023): 3375. https://doi.org/10.3390/ijms24043375.
- Sarzani, Riccardo, et al. "Molecular therapies in cardiovascular diseases: small interfering RNA in atherosclerosis, heart failure, and hypertension." International Journal of Molecular Sciences 25.1 (2023): 328. https://doi.org/10.3390/ijms25010328.
- Li, Wenjia, et al. "SCN5A variants: association with cardiac disorders." Frontiers in physiology 9 (2018): 1372. https://doi.org/10.3389/fphys.2018.01372.
- El Harchi, Aziza, and Oriane Brincourt. "Pharmacological activation of the hERG K+ channel for the management of the long QT syndrome: A review." Journal of Arrhythmia 38.4 (2022): 554-569. https://doi.org/10.1002/joa3.12741.
- Distributed under Open Access license CC BY 4.0, without modification.
