The Silent Assassin-siRNA's Precision Strike in Cancer Gene Therapy
Introduction to siRNA in Cancer
siRNA serves as a potent instrument for cancer research and treatment because it delivers precise gene expression silencing through efficient RNA interference. Small interfering RNA (siRNA) molecules consist of double-stranded RNA fragments that measure between 21 to 23 nucleotides and function to degrade targeted messenger RNA (mRNA) molecules through RNA interference (RNAi). The siRNA mechanism targets specific oncogenes and cancer-related genes for inhibition which positions it as a promising treatment approach for cancer. The field of cancer research employs siRNA to investigate the activities of genes that promote tumor growth and advancement. Through selective gene knockdown researchers uncover cancer biology roles and discover potential therapeutic targets. Research studies demonstrated that siRNA directed against the KRAS oncogene decreases tumor growth in models of pancreatic cancer. Researchers have used siRNA to target mutant versions of the p53 tumor suppressor gene that often undergo alterations in cancer cases. Researchers found that specific siRNAs targeting mutant proteins can silence those mutations without influencing wild-type proteins which restores regular cellular activities and enhances cancer cell sensitivity to treatments.
Mechanisms of siRNA in Cancer Cells
- Basic Mechanisms
As a double-stranded RNA molecule consisting of 21 to 23 nucleotides siRNA produces its effects through post-transcriptional processes. RNAi begins with the identification and cutting of long double-stranded RNA molecules. Dicer molecule identifies and attaches to extended double-stranded RNA molecules including synthetic siRNA and precursor miRNA. The siRNA duplex structure consists of two strands, namely the passenger (sense) strand and the guide (antisense) strand. The dicer-processed siRNA fragments move into the RNA-induced silencing complex (RISC) during the loading step. The RISC complex splits the siRNA strands and integrates the strand with the more stable 5'-end into the active RISC complex. AGO2 endonuclease within the RISC complex targets the passenger strand of the siRNA for cleavage which triggers duplex unwinding and destroys the passenger strand. The guide strand known as the antisense strand stays bound to the RISC. The final step involves the siRNA-loaded RISC complex searching cellular mRNA molecules to locate one that matches the siRNA guide strand. After locating a complementary target mRNA the RISC complex triggers specific site cleavage which results in mRNA degradation and gene expression inhibition.
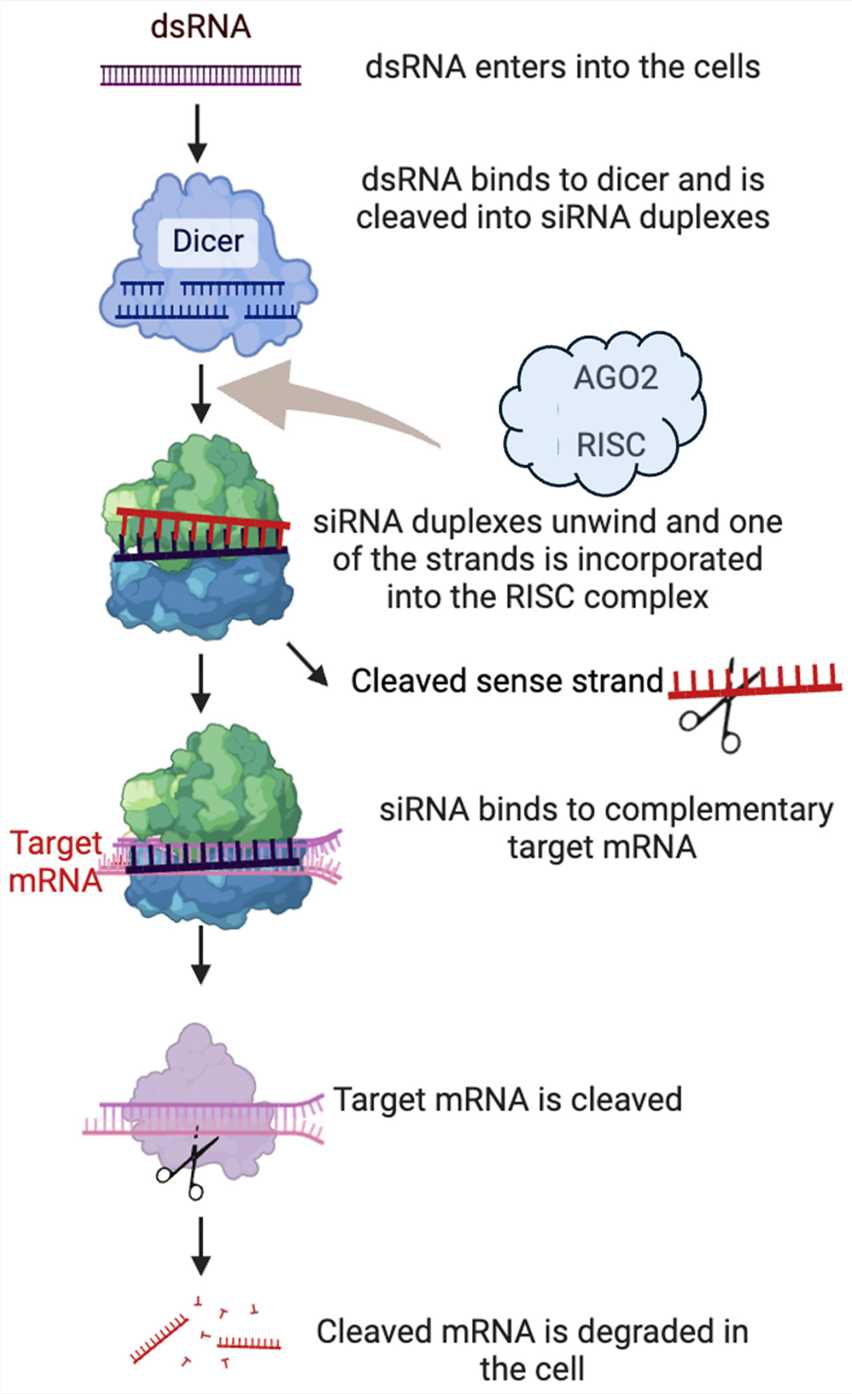 Fig. 1 Gene silencing mechanisms of RNAi1,6.
Fig. 1 Gene silencing mechanisms of RNAi1,6.
- Specific Targeting of Oncogenes
In cancer treatment, siRNA functions primarily by targeting specific oncogenes. Genes become oncogenes when they mutate or produce too much protein which leads to cancer growth and advancement. The design of siRNA molecules allows them to bind specific oncogenes to lower their activity which results in tumor growth inhibition. The use of siRNA against the c-Myc oncogene leads to reduced cell growth and induces cell death in various cancer cell lines. Research demonstrated that siRNA directed at the EGFR gene expression which is common in breast and lung cancers produces potent antitumor outcomes. Research shows that siRNA serves as an effective cancer therapy strategy because it targets and silences oncogenes.
- Inhibition of Tumor Suppressor Genes
The primary aim of using siRNA in cancer therapy is oncogene targeting yet it has the ability to modify tumor suppressor gene expression levels. Tumor suppressor genes stop cancer progression by controlling cell growth and maintaining both apoptotic processes and DNA repair functions. When tumor suppressor genes undergo functional changes or losses it promotes cancer development. siRNA therapy restores tumor suppressor gene activity by repressing their corresponding negative regulators and compensatory pathways. Experimental studies have shown that siRNA aimed at the MDM2 gene which inhibits p53 tumor suppressor actions revives p53 activity and activates cancer cell death in specific cancer models. Researchers have utilized siRNA to target DNA damage repair genes such as PARP which increases cancer cells' vulnerability to radiation and chemotherapy treatments.
Delivery Challenges and Innovations
- Systemic delivery: Lipid nanoparticles (LNPs) optimized for tumor accumulation
The systemic administration of siRNA is crucial for cancer therapy but faces limitations due to its rapid clearance by the reticuloendothelial system and susceptibility to enzymatic degradation. LNPs represent an advanced delivery system that effectively addresses present medical delivery obstacles. LNPs protect siRNA from degradation by encapsulating it within a protective lipid bilayer which also extends its circulation time. These nanoparticles use the enhanced permeability and retention (EPR) effect to target and accumulate within tumor locations.
The latest LNP formulations use pH-sensitive lipids to trigger siRNA release within the acidic conditions of tumor environments which leads to better gene silencing. LNPs undergo functionalization with targeting ligands which enhances their ability to specifically target tumor cells. The introduction of folate-conjugated LNPs has demonstrated enhanced siRNA uptake by cancer cells that display the folate receptor. The development of new technologies enables better delivery performance and specificity of siRNA in preclinical models which supports the transition to clinical trials.
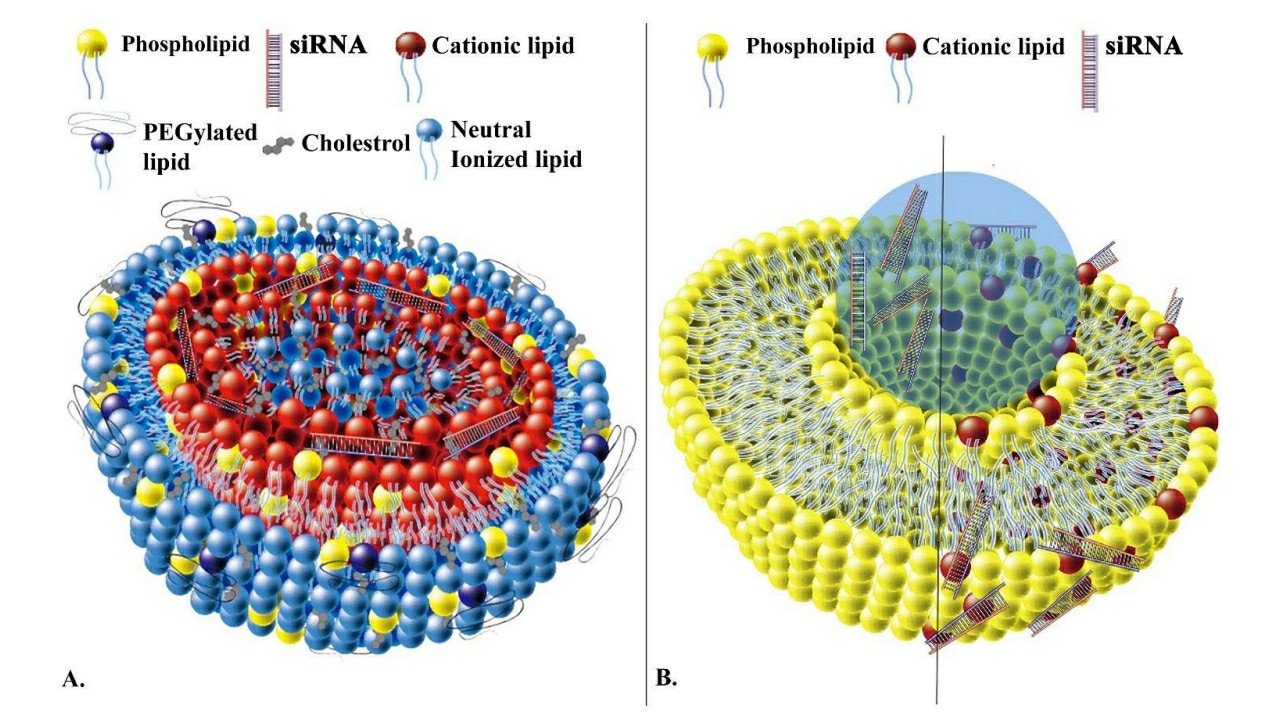 Fig. 2 LNPs for siRNA delivery2,6.
Fig. 2 LNPs for siRNA delivery2,6.
- Active targeting: Peptide-conjugated siRNA
Active siRNA delivery to targeted tumor cells stands as a pivotal advancement in therapeutic siRNA technology. The strategy involves attaching siRNA to peptides that specifically target receptors which are upregulated on cancer cell surfaces. The RGD peptide binds to integrins present in various tumor types because integrins show high levels of expression in these cells. The conjugation of siRNA with RGD peptides allows for the precise targeting of tumor cells rich in integrins which improves siRNA delivery effectiveness and decreases off-target consequences. The targeted delivery approach results in higher siRNA accumulation in tumor cells and enables dose reduction that diminishs potential damage to healthy tissues. Preclinical studies show peptide-conjugated siRNA delivers substantial antitumor activity across multiple cancer models.
- Localized delivery: Intratumoral electroporation in melanoma models
Direct tumor injection of siRNA manages to overcome numerous hurdles that come with systemic delivery methods. The technique of intratumoral electroporation combines the direct injection of siRNA into tumors with electric pulses to increase cellular absorption. The application of this technique in melanoma models revealed effective tumor reduction when siRNA targeted oncogenic drivers like BRAF. The use of intratumoral electroporation results in high local siRNA concentrations which allows for effective gene silencing while eliminating the necessity of systemic delivery methods. This delivery approach benefits solid tumor treatment by reducing systemic drug distribution and lowering potential adverse effects.
- Immune evasion
Successful siRNA delivery requires immune evasion because the immune system can detect and eliminate siRNA particles which compromises their therapeutic impact. Scientists developed multiple techniques aimed at improving the stealth characteristics of siRNA delivery systems to overcome this challenge. PEGylation represents a widespread technique that attaches polyethylene glycol (PEG) to siRNA nanoparticles. PEGylation decreases immune cell recognition of siRNA which results in prolonged circulation time and decreased clearance rate. Incorporating the "don't eat me" signal CD47 into siRNA delivery frameworks represents another innovative strategy. CD47 functions as a protein that communicates to immune cells to avoid phagocytosis of the particle. The inclusion of CD47 into LNPs or alternative delivery vehicles enables siRNA to avoid immune detection and elimination which improves its stability and effectiveness.
Preclinical Proof-of-Concept Studies
- Glioblastoma
GBM stands out as an aggressive brain tumor that grows quickly and usually leads to a poor prognosis. The EGFRvIII mutation occurs frequently in GBM tumors and presents itself as a valuable target for therapeutic treatments. Research using orthotopic mouse models demonstrates that survival increases significantly when siRNA targeting EGFRvIII is combined with temozolomide (TMZ) in preclinical settings. The studies administered siRNA targeting EGFRvIII into cells using lipid nanoparticles (LNPs) to achieve efficient tumor cell uptake. EGFRvIII siRNA combined with TMZ showed a synergistic treatment response through oncogene silencing by the siRNA and enhanced therapeutic action from TMZ. The treatment combination extended median mouse survival by about 20 days relative to control groups which indicates its possible effectiveness for GBM treatment improvement.
- Breast cancer
HER2 serves as a fundamental factor in both the development and progression of breast cancer especially when the cancer reaches metastatic stages. Research models for metastatic breast cancer have tested combined treatment with HER2 siRNA and docetaxel to improve therapy effectiveness. HER2 siRNA was packaged into nanoparticles which were engineered to release the siRNA inside cells to achieve efficient gene silencing in these models. The combination of docetaxel with the co-delivery system resulted in substantial decreases in both tumor burden and metastatic spread. When combined treatments were used, tumor volume dropped by 60% relative to docetaxel treatment alone and metastatic lesions in both lungs and liver decreased by 40%. Research findings show that siRNA-based treatments can enhance the performance of existing chemotherapy drugs in treating metastatic breast cancer.
- Ovarian cancer
FAK functions as a vital protein in cell movement and invasion mechanisms which become irregular in ovarian cancer. Research involving the ID8 mouse model of ovarian cancer demonstrated that FAK-targeting siRNA effectively decreases peritoneal dissemination which is a frequent and fatal characteristic of ovarian cancer. Researchers delivered FAK siRNA to ovarian cancer cells through a targeted nanoparticle system to achieve high cellular uptake. The treatment achieved a 70% decrease in peritoneal tumor burden when compared with control groups. The median survival time of treated mice increased by about 30 days demonstrating FAK siRNA's potential to enhance ovarian cancer treatment outcomes. These findings indicate that FAK siRNA represents a viable approach to lessen metastatic progression and extend survival rates in ovarian cancer patients.
Overcoming Resistance and Toxicity of siRNA
- Off-target effects: Chemical modifications
The effectiveness of siRNA therapy is limited by off-target effects that lead to unwanted gene silencing and adverse side effects. Scientists created chemical changes to improve siRNA treatment precision and performance. The 2'-O-methyl modification involves adding a methyl group to the ribose sugar's 2' position within siRNA molecules. The modification reduces off-target effects through stabilization of the siRNA duplex structure which prevents unintended mRNA interactions. The application of 2'-O-Me modification to the guide strand at position 2 leads to diminished off-target effects while maintaining siRNA activity.
The phosphorothioate (PS) linkage stands out as a crucial modification since it substitutes an oxygen atom with a sulfur atom within the phosphate backbone. The chemical modification grants siRNA resistance against nucleases while reducing off-target effects through degradation prevention and nonspecific binding reduction. PS modifications applied to the antisense strand's 3'-terminus significantly increase siRNA stability by extending its serum half-life from minutes to multiple days. The effectiveness of siRNA therapy grows considerably through chemical modifications which enhance specificity and reduce off-target effects.
- Stromal barriers
Delivery of siRNA to solid tumors depends on breaking through dense stromal barriers that stop drug penetration. Tumor microenvironments contain elevated levels of hyaluronic acid (HA) that helps establish dense fibrotic stromal tissue by functioning as an extracellular matrix component. Scientific studies indicate that hyaluronidase reduces stromal density by breaking down hyaluronic acid which enables siRNA to penetrate deeper into tissue layers. Combining hyaluronidase with siRNA results in increased penetration and broader distribution of siRNA throughout tumor tissue. Pancreatic cancer cell studies revealed that simultaneous delivery of siRNA targeting oncogenic drivers with hyaluronidase enhances siRNA uptake by tumor cells and improves treatment outcomes. This method shows promise to penetrate physical barriers in the tumor microenvironment to enhance siRNA transfer to target cells.
- Adaptive resistance: Epigenetic priming with HDAC inhibitors
Cancer treatment faces substantial challenges because tumor cells develop protective methods against therapeutic drugs to achieve adaptive resistance. Histone deacetylase (HDAC) inhibitors employed as epigenetic priming agents serve to overcome adaptive resistance encountered during siRNA therapy. HDAC inhibitors alter cancer cell epigenetic profiles making them more receptive to siRNA-based gene silencing treatments. Histone acetylation along with other epigenetic modifications affects both gene expression levels and cellular reactions to therapeutic interventions. HDAC inhibitors can increase histone acetylation in cancer cells which prepares them to receive siRNA more effectively and achieve stronger gene silencing. Scientific studies indicate that pretreatment with HDAC inhibitors improves siRNA effectiveness against oncogenes in multiple cancer models such as breast and ovarian cancer. The combined method exploits the combined effects of epigenetic modulation with siRNA treatment to defeat adaptive resistance while enhancing treatment results.
Advantages of siRNA for Cancer Therapy
RNAi encompasses three types of dsRNAs capable of inducing gene silencing: Three RNA types induce gene silencing in RNAi including short hairpin RNA molecules (shRNA), naturally occurring small RNA molecules (miRNA), and small interfering RNA (siRNA). Synthetic siRNAs stand out as the optimal candidate for drug development because their unique RNAi-based mechanism gives them distinct benefits over traditional chemotherapeutic drugs and other cancer treatments. SiRNA drugs demonstrate superiority through their precise gene-silencing ability. siRNAs that are 20 nucleotides long achieve high target gene specificity and minimal off-target effects through their base complement pairing recognition mechanism. Second, the exceptional safety profile. siRNAs achieve gene silencing through post-transcriptional mechanisms solely within the cytoplasm, which blocks their access to the nucleus and genome integration and therefore reduces the likelihood of host gene mutations. Third, the remarkable efficiency. Cells demonstrate substantial gene silencing effects when exposed to minimal siRNA fragments. Fourth, the unlimited number of potential targets. Developments in molecular biology together with whole genome sequencing technologies have enabled the creation of extensive human genomic databases as well as cDNA and disease gene databases. The design of siRNAs from target gene mRNA sequences enables researchers to create siRNAs that can silence disease-associated genes effectively.
Future Directions of siRNA
Combining siRNA that targets oncogenic drivers such as VEGF or survivin with chemotherapy or targeted therapies produces stronger tumor suppression effects and minimizes drug resistance in cancer treatment. Combining siRNA therapies with other treatments enhances therapeutic results while expanding the potential uses of siRNA in treating complex medical conditions. SiRNA therapies gain substantial potential through personalized medicine because treatments can be customized according to unique individual genetic profiles. Next-generation sequencing advancements together with transcriptomic analysis improvements help detect genetic vulnerabilities unique to patients which leads to the creation of targeted siRNA therapies. Patients who have BRCA mutations may see therapeutic advancements through combined treatments of PARP inhibitors and siRNA agents that target DNA repair genes such as PARP1 or RAD51. Liquid biopsy techniques allow real-time tumor gene expression monitoring which enables clinicians to dynamically adjust siRNA targets and drug combinations to maintain therapeutic effectiveness and reduce resistance. The integration of siRNA into personalized medicine strategies enhances therapeutic precision which results in improved patient outcomes and fewer side effects.
References
- Ebenezer, Oluwakemi, et al. "Recent Update on siRNA Therapeutics." International Journal of Molecular Sciences 26.8 (2025): 3456. https://doi.org/10.3390/ijms26083456.
- Ali Zaidi, Syed Saqib, et al. "Engineering siRNA therapeutics: challenges and strategies." Journal of Nanobiotechnology 21.1 (2023): 381. https://doi.org/10.1186/s12951-023-02147-z.
- Ubby, Ifeoma, et al. "Cancer therapeutic targeting using mutant–p53-specific siRNAs." Oncogene 38.18 (2019): 3415-3427. https://doi.org/10.1038/s41388-018-0652-y.
- Sajid, Muhammad Imran, et al. "Overcoming barriers for siRNA therapeutics: from bench to bedside." Pharmaceuticals 13.10 (2020): 294. https://doi.org/10.3390/ph13100294.
- Neumeier, Julia, and Gunter Meister. "siRNA specificity: RNAi mechanisms and strategies to reduce off-target effects." Frontiers in Plant Science 11 (2021): 526455. https://doi.org/10.3389/fpls.2020.526455.
- Distributed under Open Access license CC BY 4.0, without modification.
