Understanding Gene Therapy- A Journey into the Future of Medicine
Definition of Gene Therapy
Gene therapy stands as a revolutionary medical approach which utilizes the addition, alteration, or replacement of genetic material (GM) in cells to treat medical conditions. The emerging medical area of gene therapy targets genetic defects to treat genetic disorders at their root level. Traditional treatments focus on alleviating disease symptoms, while gene therapy provides enduring solutions by correcting the genetic mutations that cause the disease. Medical professionals use gene therapy to address numerous health conditions such as inherited genetic disorders and acquired diseases throughout a person's life in addition to certain cancer types. Therapeutic GM includes functional gene replacements for defective genes RNA molecules that regulate gene activity and CRISPR/Cas9 systems for correcting genetic mutations. Scientific advancements in molecular biology and genetic engineering, together with improved delivery technology, have enabled scientists to create therapeutic genes with increased precision and efficiency. Despite being a developing scientific area, gene therapy shows successful results in treating genetic conditions including certain types of inherited blindness severe combined immunodeficiency and specific muscular dystrophy forms.
What is the Goal of Gene Therapy
The main objective of gene therapy is to develop superior treatments for genetic disorders through direct modification of their foundational genetic mutations. Gene therapy aims to repair the underlying genetic defects rather than merely alleviating symptoms to allow the body to operate normally. Monogenic disorders like cystic fibrosis and hemophilia receive therapeutic intervention from gene therapy through the delivery of working gene copies that restore protein function and lessens disease symptoms in patient cells. Therapeutic advantages of gene therapy exist for both inherited genetic conditions and non-genetic acquired diseases. Cancer treatment sees benefits from gene therapy when genes are delivered to enhance immune cells' effectiveness against cancer. High-risk individuals with genetic predispositions to diseases can prevent disease onset through gene therapy treatments which address their genetic vulnerabilities. Gene therapy serves as a proactive treatment that corrects or compensates for defective genes prior to symptom manifestation. The goal of gene therapy is to enhance patient quality of life through lasting therapeutic improvements while providing possible cures for currently only symptom-managed conditions.
Types of Gene Therapy
- Somatic Cell Gene Therapy
The process of somatic cell gene therapy targets non-reproductive cells of the body to implement genetic alterations. This treatment method targets distinct diseases by modifying somatic cells so that genetic alterations impact solely the treated individual and do not pass to next generations. Somatic cell gene therapy can be performed using two main approaches: ex vivo and in vivo.
Ex Vivo approach
Researchers extract cells from patients to perform genetic engineering in the laboratory before they reintroduce the modified cells back into the body. Patients with blood disorders such as severe combined immunodeficiency (SCID) and certain types of anemia experience substantial treatment benefits from this therapeutic approach. Healthcare professionals transplant hematopoietic stem cells (HSCs) which researchers modify genetically using lentiviral or retroviral vectors. Clinical research studies have validated this treatment method because it demonstrates patient healing capabilities and enhanced quality of life for particular diseases.
In Vivo approach
In vivo gene therapy applies direct injections of genetic materials into cells inside living organisms to produce therapeutic results. Both adenovirus (AV) and adeno-associated virus (AAV) vectors serve essential roles in scientific experiments to transport therapeutic genes to specific tissue targets. Scientific studies have evaluated in vivo gene therapy as possible treatments for medical conditions like CF muscular dystrophy and specific kinds of visual impairments. AAV vectors enable doctors to insert functional CFTR genes into CF patients' airway epithelial cells thereby restoring normal chloride channel function. Although preclinical research and initial clinical trials demonstrate promise for the method it still encounters difficulties in the precise and effective delivery of therapeutic genes to target locations.
- Germline Gene Therapy
Germline gene therapy induces permanent genetic alterations within reproductive cells or early embryos which then transfer to all future generations. Germline gene therapy achieves the elimination of hereditary genetic disorders by performing specific mutation corrections within reproductive cells. The importance of ethical and safety concerns associated with germline gene therapy grows because any genetic modification applied through this technique will affect every generation descended from the treated individual.
Germline gene therapy causes extensive debate because its genetic modifications affect the human gene pool across several generations without possibility of reversal. Ethical worries regarding germline gene therapy encompass unforeseen outcomes and genetic disparities among humans and its extensive influence on human evolutionary development. In response to these concerns multiple nations have chosen to completely ban germline gene therapy or establish strict regulatory controls. CRISPR/Cas9 genome-editing methods enable researchers to conduct germline gene therapy with high effectiveness. Scientists use genome-editing tools to precisely modify DNA sequences and correct specific genetic mutations. The CRISPR/Cas9 system introduces substantial hazards for germline modification because it creates genetic alterations that affect only particular cells due to off-target effects and mosaicism.
Gene Therapy utilizes both Viral and Non-viral Vectors as Delivery Systems
- Viral Vectors
Medical treatments benefit from viral vectors which scientists develop by modifying dangerous viruses to become safe. These vectors achieve efficient delivery of GM into target cells while maintaining gene expression for long durations. Adenovirus, AAV and lentivirus represent the most commonly used viral vectors in medical treatments. AAV vectors show promise in treating CF through the delivery of functional CFTR genes into the airway epithelial cells. Viral vectors pose safety risks due to their potential to trigger immune responses and cause insertional mutagenesis when viral DNA joins the host genome disrupting normal gene function.
Retroviral vectors
Retroviral vectors serve as common gene delivery tools for both somatic and germline gene therapies. Retroviral vectors differentiate themselves from adenoviral and lentiviral vectors by penetrating nuclear pores during mitosis which enables transfection of dividing cells and establishes them as suitable for in situ treatment. The generation of vectors through complete viral gene elimination produced approximately 8 kb of space that could be used for transgenic insertion. Despite their linear integration capability within host cell genomes that makes retroviruses excellent for somatic cell ex vivo delivery they have led to both successful X-SCID gene therapy treatments and leukemia development in some patients because retroviral DNA integration into the LMO2 gene causes its inappropriate activation. Retroviral vectors have been utilized in gene therapy treatments for familial hyperlipidemia and tumor vaccinations. The major challenges of retroviral vectors include inefficient delivery in live organisms combined with immunogenic responses which prevent them from transducing non-dividing cells while posing risks of activating oncogenes or disabling tumor-suppressor genes during insertion.
Adenoviral vectors
Research teams extracted adenoviral vectors from numerous species and identified more than one hundred different serotypes. The majority of adults have been exposed to adenovirus serotypes 2 and 5 which represent the most commonly employed vectors in gene therapy. The ability of adenoviruses type 2 and 5 to deliver genes across diverse tissues stems from their minimal host specificity. The adenovirus vectors deliver DNA particles that reach up to 38 kb while their gene expression is limited to a short duration due to their inability to integrate into the host genome. Natural and acute immune responses against adenoviruses restrict their therapeutic use to limited tissue types. While natural adenovirus infections typically do not cause serious illness and their GM stays separate from host chromosomes, adenoviral vectors used in gene therapy have caused serious health problems and even death in some patients. Scientists created a safer adenoviral vector through gene deletions that halt viral replication and extensive genome removal to house 38 kb of transgene particles leading to "gutless" or "pseudo" adenoviruses.
Adeno-associated vectors
AAV vectors demonstrate similar characteristics to adenoviral vectors but their limited replication ability and pathogenicity create safer alternatives. AAVs have no capacity to cause human diseases. AAV vectors demonstrate a unique characteristic by integrating specifically into chromosome 19 to enable long-term expression and avoid negative effects within living organisms. These vectors face significant limitations due to their complicated production methods and their transgene capacity limitation of just 4.8 kb. Scientists have started using AAV vectors to treat disorders including cystic fibrosis (CF), hemophilia B, Leber congenital amaurosis, and Alpha-1 antitrypsine deficiency.
Herpes simplex virus vectors
The HSV system is among the newest viral vectors under consideration for gene delivery applications. In HSV systems disabled infectious single copy (DISC) viruses are produced which possess a glycoprotein H defective mutant HSV genome. The defective HSV transforms complementing cells into factories that produce viral particles leading to permanent infection because its genome replicates without forming new infectious particles. The HSV vector system can transport as much as 150 kb transgenic DNA while its neuronotropic characteristics establish it as the top vector for gene delivery to both nervous system cells and cancerous tumor cells.
Lentivirus vectors
Lentiviruses are a subclass of retroviruses. Lentiviruses have been adopted as gene delivery vectors recently because they can integrate with nondividing cells which distinguishes them from other retroviruses that infect only dividing cells. Lentiviral vectors have the ability to transport genetic information up to 8 kb in length. Lentiviruses effectively target neural stem cells and are excellent for ex vivo gene transfer in the central nervous system because they do not provoke major immune reactions or adverse effects. Lentiviral vectors provide efficient infection of both dividing and nondividing cells with stable transgene expression over time and low immunogenicity while being capable of carrying large transgenes.
Poxvirus vectors
The Poxviridae family includes poxvirus vectors which serve as effective systems for high-level cytoplasmatic transgene expression. The most beneficial characteristic of this virus for gene delivery lies in its high stable insertion capacity which exceeds 25 kb. The process of transgene sequence insertion into vaccinia virus vectors stands apart from other vector systems because it employs homologous recombination or in vitro ligation to create recombinant vectors. Different studies have employed poxviruses to treat prostate cancer along with colorectal cancer breast cancer and lung cancer. Expression of human papilloma virus types 16 and 18 E6 and E7 genes occurred through recombinant vaccinia virus vectors in cervical cancer patients to promote tumor regression.
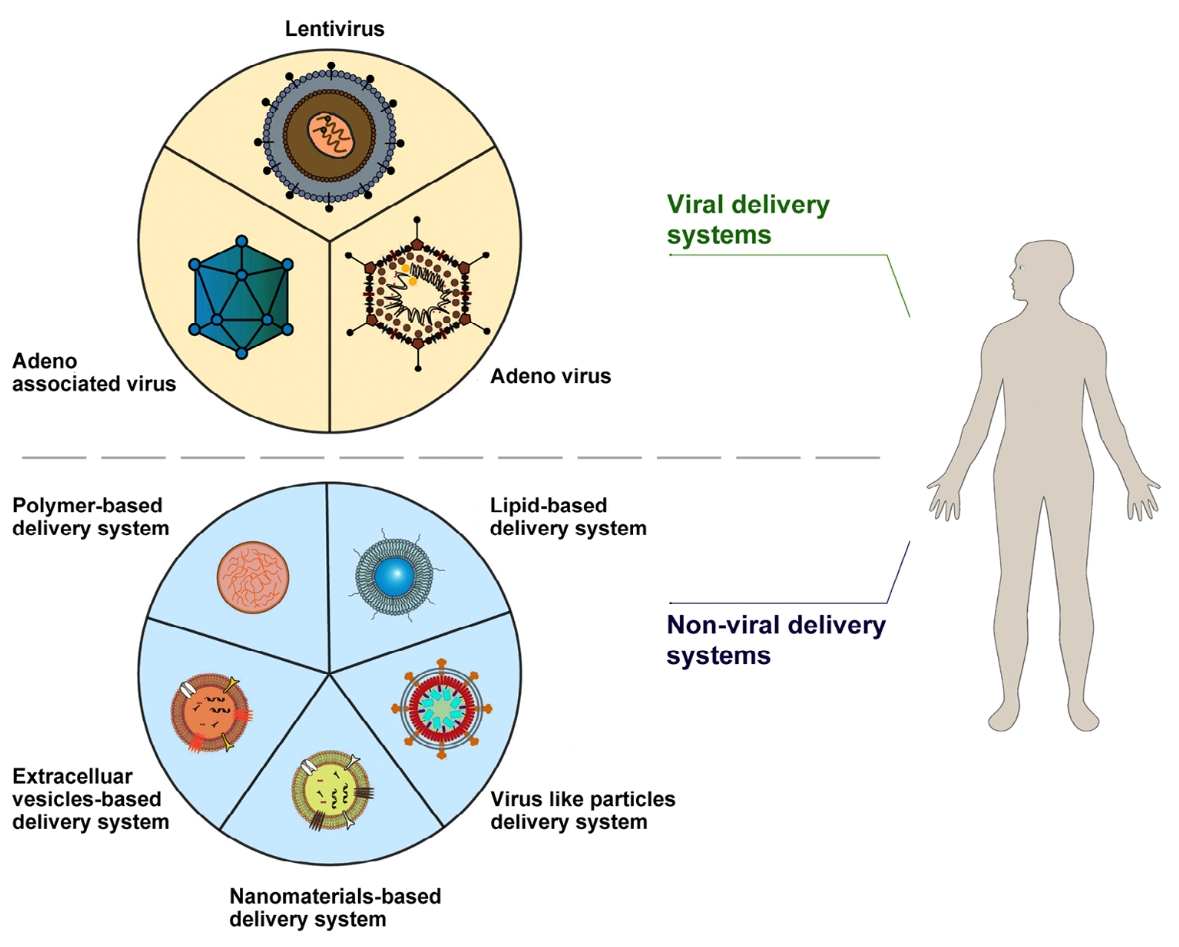 Fig. 1 Gene therapy delivery systems are categorized as viral system and nonviral system1,6.
Fig. 1 Gene therapy delivery systems are categorized as viral system and nonviral system1,6.
- Non-Viral Vectors
Gene delivery non-viral vectors consist of plasmid DNA in conjunction with nanoparticles and liposomes. These vectors generate safer results by limiting immune responses but need greater amounts to reach effective gene delivery due to lower transduction abilities. Developing nanoparticles with enhanced capabilities for cellular entry and endosomal escape stands as a key progress in non-viral vector technology used for gene therapy. Liposomal nanoparticles deliver chemically modified CFTR mRNA which restores chloride channel function in cystic fibrosis patients.
Naked DNA
Skin tissue and thymus tissue along with cardiac muscle accept GM through direct naked DNA injection which works best for transferring genes into skeletal muscle and liver cells. The skeletal muscle maintained active gene expression beyond the 19-month mark after receiving the injection. A single injection leads to transgenic expression in less than 1% of muscle myofibers but repeated injections boost expression levels. While the injection technique for naked DNA presents a safe and simple approach its gene delivery effectiveness is inadequate which limits its application to DNA vaccination purposes.
Nanoparticles
GM finds protection from degradation through nanoparticle encapsulation. Targeting ligands functionalization of nanoparticles enhances delivery precision for specific cell types which improves overall efficiency. Compared to viral vectors nanoparticles demonstrate lower immunogenicity. To obtain effective cellular uptake and endosomal escape nanoparticles demand optimization. Producing nanoparticles presents a more intricate and expensive process than generating plasmid DNA.
Liposomes
Liposomes present biocompatible characteristics and deliver their payload through controlled release mechanisms. Liposomes generate fewer immune responses than viral vectors and possess the ability to direct delivery to particular tissues. Liposomes show poor transduction performance and lack stability within biological environments which can result in early GM release.
Exosomes
Exosomes serve as naturally occurring nanoscale extracellular vesicles that carry materials between cells to enable communication with both nearby and distant cells after their release from the originating cells. Because they work as endogenous carriers for intercellular material transportation, they have been utilized to transport GM, which makes them potential vectors for in vivo gene therapy. Exosomes have been shown to transport human CFTR mature glycoprotein and CFTR mRNA to Chinese hamster ovarian cells and CFTR-deficient cells from CF patients. Functional improvements in CFTR channels were observed in cells that received exosomes in both tested models. Exosomes demonstrate their ability to deliver small interfering RNA in well-differentiated human airway epithelial cells while indicating their usefulness for transporting gene editing materials beyond traditional gene transfer. Researchers showed that exosomes could function as vectors to deliver CFTR to human CF cells which led to functional correction of genetic defects.
Gene Therapy in Action: Cystic Fibrosis as a Case Study
- Application of Gene Therapy in Cystic Fibrosis (CF)
The CFTR gene mutations trigger CF, a deadly genetic disorder that disrupts chloride ion transport regulation through a membrane-bound protein. The genetic defect leads to the production of thick mucus which accumulates in lungs and other organs and results in persistent infections along with progressive damage to various organs. CF gene therapy works by delivering the functional CFTR gene to the damaged airway epithelium cells to correct the genetic defect. CF gene therapy research began in the 1990s and scientists have evaluated multiple methods through preclinical and clinical trials. Researchers developed an effective method using AAV and lentivirus viral vectors to transport the CFTR gene into airway epithelial cells. The inhaled lentiviral vector can deliver a complete CFTR gene to CF model animal lungs. Preclinical studies have demonstrated the effectiveness of this method because 9-15% of respiratory epithelial cells expressed CFTR. Researchers apply non-viral delivery methods including liposomal nanoparticles (LNPs) to transport chemically altered CFTR mRNA to airway epithelial cells. CF animal models have shown restored chloride channel function with this method which allows repeated dosing without triggering major immune responses.
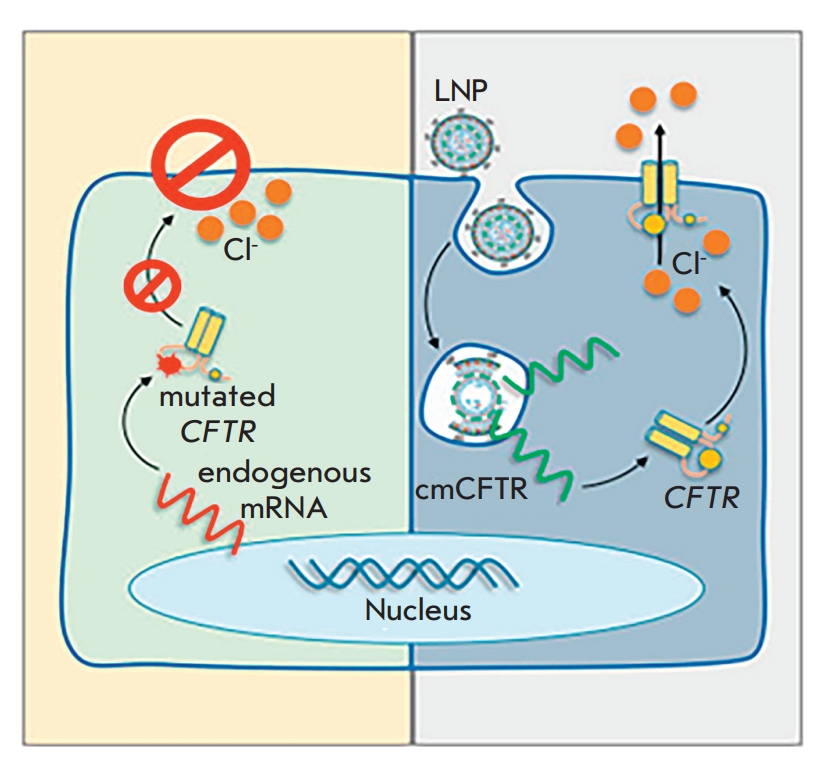 Fig.2 LNP-cmCFTR delivery2,6.
Fig.2 LNP-cmCFTR delivery2,6.
- Challenges Specific to Cystic Fibrosis
The treatment of CF with gene therapy involves multiple obstacles due to the disease's specific characteristics and the affected tissues. Animal models of CF encounter a significant challenge due to the thick mucus layer blocking access to target areas for therapeutic genes. The mucus obstructs both viral and non-viral vectors from penetrating target tissues which lowers the effectiveness of gene transfer methods. Researchers have created inhaled delivery systems to enable vectors to reach airway epithelial cells more effectively as a solution to this challenge. Preclinical studies have utilized an optimized inhaled lentiviral vector specifically designed to improve gene transfer efficiency into airway epithelial cells. Viral vector-based treatments face substantial challenges from adverse immune responses. The immune system attacks viral vectors because it recognizes them as threats which diminishes their effectiveness and could cause harmful side effects. Scientists developed numerous methods to reduce viral vector immunogenicity to solve this particular issue. Researchers discovered that AAV vectors with modified capsids can avoid detection by the immune system which allows for multiple doses. Research into non-viral vectors like LNPs continues because these vectors typically produce lower immune reactions than viral vectors.
The Promise and Potential of Gene Therapy
- Potential of Gene Therapy to Treat a Wide Range of Genetic Disorders
Gene therapy represents a groundbreaking medical solution for numerous genetic disorders because it directly addresses and repairs the underlying genetic defects rather than simply treating the symptoms. This method produces lasting treatment outcomes for disorders resulting from both singular genetic mutations and intricate genetic interactions. Monogenic disorders that result from single-gene mutations display exceptional potential for successful gene therapy interventions. Patients with sickle cell disease who possess β-globin (HBB) gene mutations now experience considerable health improvements thanks to progress in gene therapy methods. Functional HBB genes were delivered to hematopoietic stem cells through lentivirus vectors which generated healthy red blood cells and reduced patients' need for blood transfusions. Gene therapy shows potential in treating hemophilia by delivering functional gene copies that address clotting problems from F8 or F9 gene mutations.
- Ongoing Research and Future Potential of Gene Therapy
The field of gene therapy sees rapid advancements as researchers conduct multiple clinical trials and studies to develop potential treatments for different diseases. CRISPR/Cas9 represents one of the most intriguing research frontiers currently explored in scientific research. The application of CRISPR/Cas9 technology enabled scientists to repair genetic defects in human hematopoietic stem and progenitor cells that lead to SCD and this breakthrough offers potential for developing a future cure. Scientists investigate CRISPR technology's potential to treat genetic conditions such as Duchenne muscular dystrophy and CF. Scientific investigators in biomedicine focus their efforts on creating improved delivery methods for gene therapy treatments. Researchers focus on enhancing AAV and lentivirus viral vectors to boost performance and reduce immune responses. Non-viral delivery systems such as nanoparticles and liposomes are under scientific investigation due to their potential to transport GM with minimized immune responses and increased capacity for genetic payloads. The development of vector technology significantly expands gene therapy possibilities for a wide range of diseases and patient demographics.
- The Road Ahead: Future Developments in Gene Therapy
Gene therapy advancement relies on emerging advancements in gene-editing technologies such as CRISPR/Cas9 and its derivatives. CRISPR/Cas9 has revolutionized the genome editing field through its ability to perform DNA manipulation with high precision and efficiency. This technology empowers scientists to modify specific DNA sequences enabling the correction of genetic mutations that lead to diseases. The therapeutic possibilities of CRISPR/Cas9 for monogenic diseases have been demonstrated through the correction of sickle cell anemia mutations in CD34+ cells derived from patients. Scientists are expanding their gene-editing exploration beyond CRISPR/Cas9 with new techniques such as base editing and prime editing. Base editing allows one DNA base to convert to another base directly without generating double-strand breaks which minimizes potential off-target effects. Prime editing allows for accurate insertion of new GM at specific DNA sites which makes it a flexible tool for fixing various genetic defects. Modern advancements in gene-editing technologies expand gene therapy applications and improve treatment options for intricate genetic diseases.
References
- Liu, F.; Li, R.; Zhu, Z.; Yang, Y.; Lu, F. Current developments of gene therapy in human diseases. MedComm. 2024, 5:e645. https://doi.org/10.1002/mco2.645.
- Lomunova M.A.; Gershovich P.M. Gene therapy for cystic fibrosis: recent advances and future prospects. Acta Naturae (англоязычная версия). 2023, 15(2): 20-31. https://doi.org/10.32607/actanaturae.11708.
- Gonçalves, G.A.R.; Paiva, R.M.A. Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo). 2017, 15: 369-375. https://doi.org/10.1590/S1679-45082017RB4024.
- Sui, H.; Xu, X.; Su, Y.; Gong, Z.; Yao, M.; Liu, X.; Zhang, T.; Jiang, Z.; Bai, T.; Wang, J.; Zhang, J.; Xu, C.; Luo, M. Gene therapy for cystic fibrosis: Challenges and prospects. Front. Pharmacol. 2022, 13:1015926. https://doi.org/10.3389/fphar.2022.1015926.
- Maule, G.; Arosio, D.; Cereseto, A. Gene Therapy for Cystic Fibrosis: Progress and Challenges of Genome Editing. Int. J. Mol. Sci. 2020, 21, 3903. https://doi.org/10.3390/ijms21113903.
- Distributed under Open Access license CC BY 4.0, without modification.
