The Delivery Conundrum-Solving siRNA's Last Mile Problem
Definition of RNAi and siRNA
RNA interference (RNAi) describes the cellular mechanism that selectively silences gene expression. The phenomenon was first discovered in 1998. RNA interference operates as a posttranscriptional gene silencing technique that digests messenger RNA (mRNA). Dicer endonuclease processes double-stranded RNA (dsRNA) into 21-23 base pair fragments called small interfering RNA (siRNA) during cytoplasmic activity. siRNA connects with helicase to create the RNA-induced silencing complex (RISC) which then targets and destroys matching mRNA strands. RNA interference represents a conserved biological process found across numerous eukaryotic species. siRNAs possess the theoretical ability to deactivate virtually any target gene through sequence alterations. Due to its specificity alongside efficiency and simplicity RNA interference establishes siRNA as the preferred tool for gene regulation. Researchers in 2001 demonstrated that siRNAs can activate RNAi in mammalian cells showing potential for siRNA use in clinical settings. The inaugural clinical trial of an siRNA drug to treat wet age-related macular degeneration began in 2004 and simultaneous evaluations took place for various other siRNA drugs to combat different diseases.
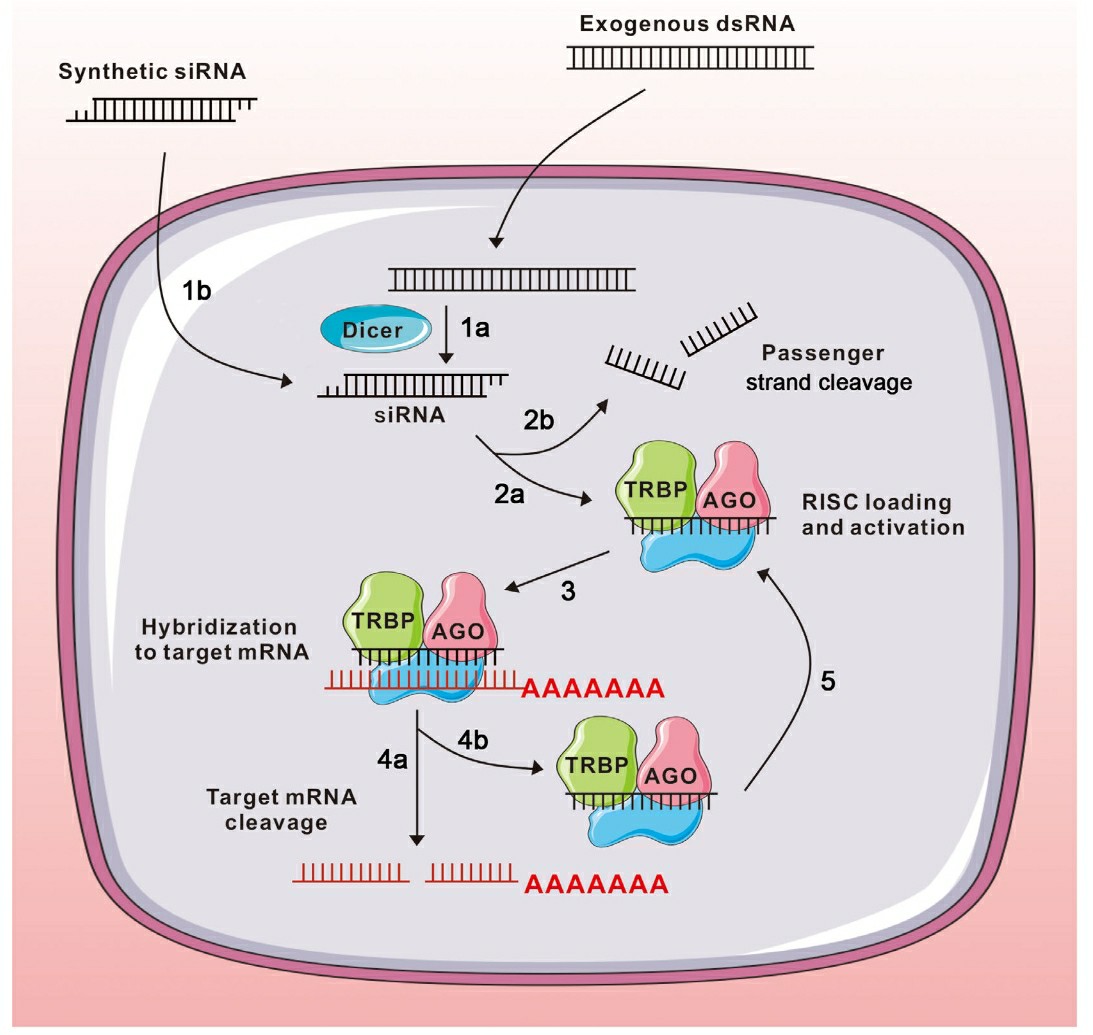 Fig. 1 RNA interference1,6.
Fig. 1 RNA interference1,6.
siRNA's Delivery Dilemma: Biological Barriers Breakdown
- Stability and targeting of siRNA
siRNA breaks down fast after entering the cytoplasm and both tissue and plasma environments. The half-life of naked siRNA in serum lasts between a few minutes up to one hour which makes it hard to accumulate enough target sites for therapeutic effectiveness. siRNA molecules are too small and negatively charged to penetrate the cell membrane, which prevents them from building up inside cells. Delivery systems that use endocytosis to transfer siRNA need mechanisms for escaping endosomes. siRNA needs to be accurately recognized and incorporated into RISC to avoid destruction when it reaches the cell cytoplasm through intracellular RNA. Modified locked nucleic acid structures have been added to siRNA complexes to enhance their stability and efficacy and address delivery challenges. Research demonstrates that sugar 2′-modifications represent the most efficient approach to boost siRNA effectiveness. The incorporation of 2′-O-methyl, 2′-fluoro, and 2′-methoxyethyl chemical modifications strengthens siRNA duplex stability and enhances binding affinity. These modifications lead to reduced activation of the innate immune system.
- Off-target effects
The RNAi approach for precise gene silencing encountered practical difficulties aside from delivery challenges. The treatment with siRNA can silence genes that were not intended as targets which is referred to as off-target gene silencing. Scientists consider off-target gene silencing hazardous because it produces damaging gene expression mutations and unexpected cell transformations. Recent research shows that off-target gene silencing occurs mainly due to seed region homology of 6 to 7 nucleotides in the siRNA sequence. Poor selection between guide and passenger strands by RISC leads to higher chances of siRNA duplexes binding to incorrect targets. The development of siRNA-based therapies demands extensive testing for all candidate siRNA sequences because off-target silencing cannot be overlooked. Scientists utilize surface-ligand modifications to achieve more precise targeted delivery. These modifications seek to address the insufficiency of the enhanced permeation and retention (EPR) effect which may lead to long-term harmful consequences through off-target effects. The latest siRNA formulation advancements now integrate antibody conjugation along with chemical modifications and receptor-targeted delivery systems to achieve better target specificity while minimizing off-target effects. Antibody conjugation with siRNA (forming antibody-siRNA complexes) demonstrates potential to minimize off-target effects.
- Immune response activation
The innate immune system quickly reacts to protect against pathogens by detecting them non-specifically and removing them from the body. The small structure of siRNA (under 30 nucleotides) prevents nonspecific activation of the interferon response by evading detection by the immune system. Subsequent research demonstrated that siRNA triggers immune responses which produce cytokines in both live organisms and laboratory settings. siRNA molecules activate the immune response directly or indirectly through cationic lipid-based delivery systems used for their administration in living organisms. Scientific investigation indicates that specific siRNA molecules trigger immune cells to generate pro-inflammatory cytokines and interferon via toll-like receptors and protein kinase R pathways. The primary cause of immune activation triggered by siRNA molecules is guanosine-cytosine (GC) rich sequences which activate numerous signaling pathways including nuclear factor kappa B (NF-κB), interferon regulatory factors and both TLR-dependent and independent pathways such as TLR7, TLR8 and TLR9. These pathways recognize siRNA as double-stranded RNA which activates inflammatory and antiviral responses.
- Intravascular degradation and renal clearance
Plasma nuclease enzymes degrade siRNA immediately following injection making it the initial biological challenge. Naked siRNA remains unstable in systemic circulation because it is highly sensitive to A-type nucleases that exist both outside and inside cells. The half-life of siRNA ranges from 10 minutes to 1 hour because renal elimination processes eliminate it quickly. The quick destruction of siRNA by tissue and plasma nucleases within minutes to hours constrains the usefulness of siRNA-based treatments. The kidney easily filters siRNA because its molecular weight stands at about 13 kDa and its length measures approximately 7 nm. siRNA modification alongside polymer conjugation as well as the use of siRNA-cationic polymer and cationic comb-type copolymers (CCC) becomes essential for protecting siRNA from nuclease degradation and enhancing its in vivo performance and therapeutic effectiveness.
- Entrapment through reticuloendothelial system (RES)
The RES system presents the most significant obstacle for nucleic acid treatments because it performs phagocytosis. Macrophages in the RES rapidly eliminate opsonized nanoparticles carrying siRNA. siRNA therapies require protection from phagocytic cells of the mononuclear phagocyte system after they enter circulation. The siRNA nanoparticles travel to RES organs with rapid speed while they remain in the bloodstream. The delayed processing and clearance of these carriers leads to their extended duration inside the organs. The RES filtration process when using siRNA treatments specifically benefits cases where the target organ has high RES content. Both the absorption process and distribution within RES can face inhibition from diverse factors such as the dimensions of the carrier particle and its electrical charge alongside surface properties. Theoretical evidence indicates that negatively charged carriers face greater elimination from the bloodstream when compared to those with positive or neutral charges.
- Impermeability of membrane
The cell membrane blocks siRNA from entering because its negative charge combined with large size and high hydrophilicity prevent passage. Effective delivery of siRNA demands modification because it faces a delivery obstacle. The negative net charge of siRNA becomes hidden when it forms complexes with cationic polymers or lipids. The negative charge of cellular membranes interacts with positively charged nanoparticles to drive internalization processes. Aptamers, ligands and immunoglobulins that target specific cell antigens serve as alternative methods to deliver siRNA effectively. When conjugates bind to cells they enable siRNA uptake through receptor-mediated endocytosis. Through this process endosomes are formed which then transport siRNA into the cytoplasm. Researchers select multiple carriers like cationic polymers and peptides for siRNA delivery because siRNA demonstrates hydrophilic properties and holds a negative charge. Scientists find peptides ideal for siRNA delivery because their broad physiochemical features and functional capacities show significant potential. Delivery applications for peptides can utilize cell-penetrating peptides (CPPs) along with non-covalent multifunctional peptide complexes and endosome-disrupting peptides.
Overcoming challenges of siRNA delivery using nanotherapeutics
An effective siRNA delivery system requires non-immunogenicity together with biocompatibility and degradable properties. Delivery strategies of siRNA need to incorporate protective measures against serum nucleases while ensuring proper delivery to target cells or tissues. Delivery methods must secure target tissue specificity after systemic administration to avoid quick removal by the liver or kidneys. The delivery vehicle must enable siRNA to move from endosomes into the cytoplasm after its internalization into target cells through endocytosis. The system enables siRNA molecules to work together with the endogenous RISC within cells. Effective non-viral delivery vehicles for siRNA must be designed to ensure cellular and tissue bioavailability together with stability. Researchers successfully developed siRNA nanotherapeutics during the past twenty years.
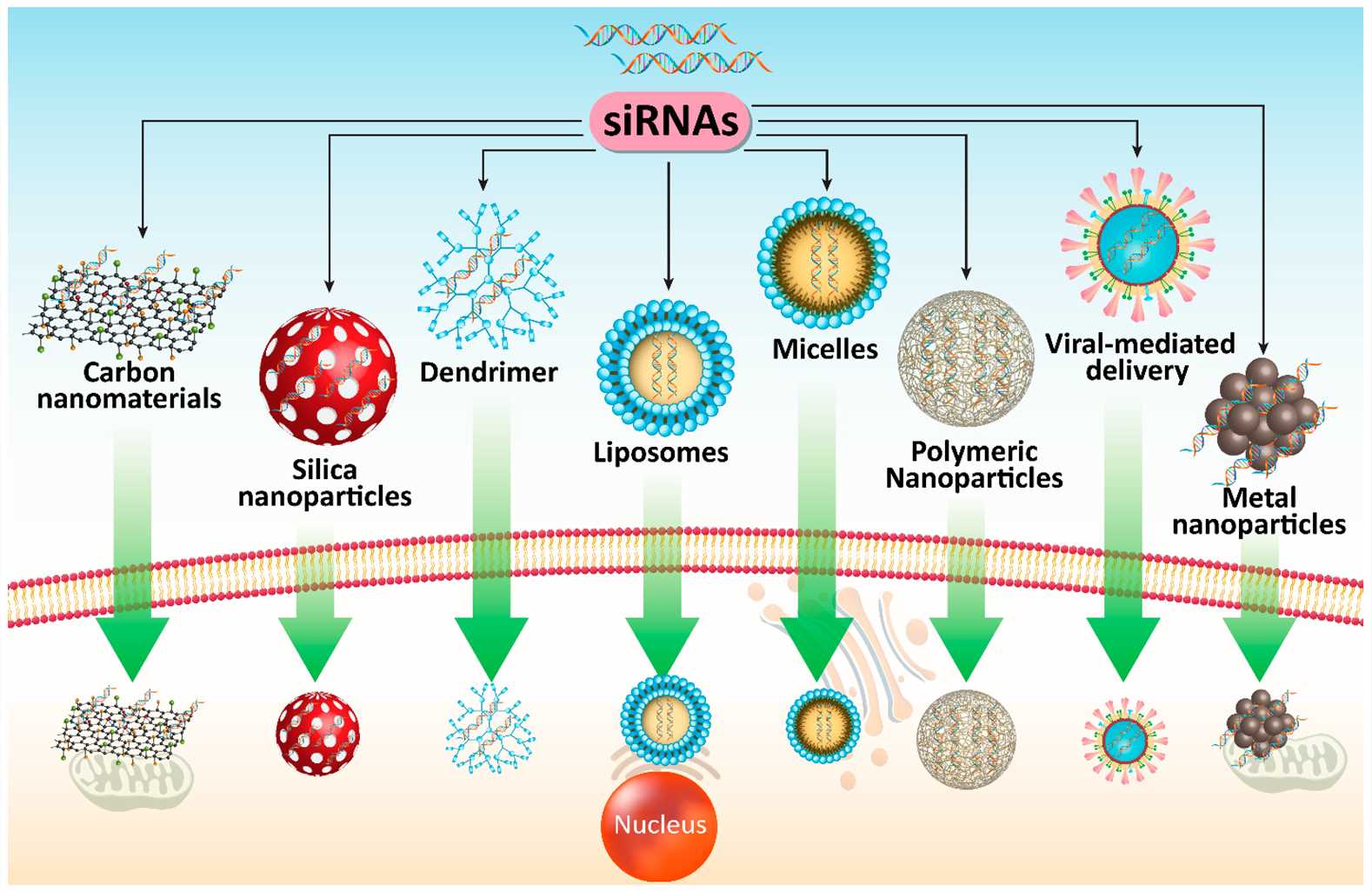 Fig.2 Different co-delivery systems for siRNA in PC therapy2,6.
Fig.2 Different co-delivery systems for siRNA in PC therapy2,6.
- Lipid-based siRNA nanotherapeutics
Lipid-based nanoparticles (LNPs) show promising capabilities for siRNA delivery because they demonstrate higher biocompatibility and exhibit reduced toxicity compared to their inorganic and synthetic counterparts. Cationic lipids are preferred for siRNA delivery systems because they demonstrate superior pharmacokinetic profiles and achieve high transfection efficiency into mammalian cells through their electrostatic interactions with nucleic acids. Scientists have explored siRNA delivery through lipid nanoparticles consisting of lipid-like molecular structures. The adaptable structure of lipids provides enhancements in their kinetic behavior and safety profile during biological applications. Lipids undergo screening to determine how their partial structural modifications influence their properties as lipidoids through minimal experimental procedures. Anderson's group completed three lipid screening studies for siRNA delivery systems throughout the past ten years. The research examined how nanoparticles delivered through intravenous injections achieved FVII suppression in mice. Scientists created C12–200 lipidoid nanoparticles which demonstrated an ED50 (0.01 mg/kg) when delivering siRNA. The team led by Whitehead improved biocompatibility and found 304O13 nanoparticles to achieve gene knockdown with an ED50 of 0.01 mg/kg while avoiding significant cytokine induction and inflammation despite administering high siRNA doses of 1 mg/kg. The peptide-based lipid library screening led to the identification of cKK-E12 nanoparticles which demonstrated superior FVII knockdown efficacy with an ED50 value of 0.002 mg/kg compared to DLin-MC3-DMA-LNP.
- Exosomes
Exosomes function as natural endogenous vesicles that transport nucleic acids and proteins and have become safe and effective siRNA delivery carriers. Exosomes demonstrate tissue-specific accumulation along with surface molecule variations based on the originating cell type that produces them. To mitigate safety risks and immunogenic responses during siRNA delivery it is beneficial to utilize exosomes generated from the recipient's personal cells. Research has demonstrated siRNA encapsulation within exosomes collected from human serum using electroporation despite exosomes being natural carriers for siRNA delivery. The ability of recipient-derived exosomes to transport siRNA to human monocytes and lymphocytes was proven through successful delivery. Scientists developed modified dendritic cells to generate exosomes that carry rabies viral glycoprotein (RVG) peptides to target neuronal cells. The incorporation of siRNA into RVG peptide-expressing exosomes achieved BACE1 gene silencing in the brain displaying their ability to traverse the blood-brain barrier to deliver therapeutic molecules to neuronal cells.
- Bioconjugate siRNA
Chemical modification of biomolecules or their incorporation into nanoparticles allows for covalent coupling with siRNA to enhance its in vivo effectiveness. Scientists can attach biomolecules like dendrimers and peptides to siRNA to enhance its delivery process. Researchers can covalently attach cell targeting peptides or bio-functional peptides to siRNA for improved delivery. The peripheral groups of PAMAM (poly amidoamine) dendrimers have been used to attach drugs covalently to increase therapeutic effectiveness and solubility while reducing toxicity and controlling drug release. The modification of poly-amidoamine dendrimer surfaces through controlled attachment of targeting ligands reduces cytotoxicity while simultaneously improving transfection efficiency and enhancing targeting ability. In a study by Choi et al. A self-crosslinked fusogenic KALA peptide together with a cell-penetrating Hph1 peptide were functionalized with siRNA that had branching PEG attached to suppress gene expression in MDA-MB-435 cell lines through polyelectrolyte complex micelles. The lung's innate immune responses were targeted for inhibition using intratracheally delivered siRNA against the p38 mitogen-activated protein kinase (MAPK) which was combined with the HIV TAT cell-penetrating peptide.
- Polymeric nanoparticles
Scientists address nucleic acid formulation limitations by encapsulating siRNA within nanoparticles which enhance serum stability and distribution while providing controlled release. Therapeutic outcomes from natural biodegradable nanoparticles have demonstrated substantial effectiveness. Natural polymers such as albumin and chitosan along with cyclodextrin gelatin and atelocollagen stand as examples. PLGA, PEI and PEG represent the synthetic polymers most commonly studied for delivering siRNA. The cyclodextrin polymer (CDP) technology served as the initial nanoparticle delivery system used in clinical trials for siRNA applications functioning as the fundamental material. Bacteria break down cellulose to create CDP. Pharmaceutical companies use this polycationic oligosaccharide to deliver small compounds. The self-assembled CPD composition including human transferrin (Tf) and PEG named CALAA-01 demonstrated enhanced capability for targeting cells within siRNA delivery systems. Scientists conducted a clinical phase experiment to evaluate this method. Nanomedicine frequently employs polymeric nanoparticles that are based on chitosan. Chitosan consists of N-acetyl-D-glucosamine (deacetylated unit) and-(1–4)-linked D-glucosamine (acetylated unit) as its main components and originates from chitin which makes it a polysaccharide. Chitosan nanoparticles possess a positive charge which enables them to interact with negatively charged siRNA through electrostatic interactions thus easing the formulation process. The orthotopic xenograft cancer model showed reduced tumor development after intratumoral injection of atelocollagen/siRNA complexes.
- Viral Vectors for siRNA: Borrowing Nature's Delivery Pros
Adeno-associated virus (AAV) vectors represent an efficient delivery system for siRNA which enables durable gene silencing with notable effectiveness. AAV vectors stand out because of their minimal immune response together with their functional ability to modify both replicating and resting cells while maintaining continuous gene expression. Therapeutic siRNA cassettes can be delivered by these vectors through tissue-specific promoters which enable precise target gene knockdown and sustained silencing. Investigators have achieved successful AAV-mediated siRNA delivery in preclinical models that target cancer cells and genetic disorders. Self-complementary AAV (scAAV) vectors boost both the potency and efficiency of siRNA delivery. AAV vectors incorporating tissue-specific promoters provide additional control mechanisms to limit siRNA expression exclusively to targeted tissue cells. By adopting this technique researchers are able to decrease unintended effects while maximizing both the safety and effectiveness of siRNA treatments. Research on hepatitis B virus (HBV) has shown that AAV vectors with siRNA sequences controlled by liver-specific promoters can effectively reduce HBV replication for up to 32 weeks in mouse models. The targeted treatment enhances therapeutic results while simultaneously lowering systemic side effects risk.
Case Studies: Delivery Success Stories
- Patisiran: How LNP delivery won FDA approval
Patisiran (Onpattro) achieved a significant breakthrough in siRNA delivery by becoming the first siRNA therapeutic to receive FDA approval. Researchers designed Patisiran to treat the polyneuropathy condition resulting from hereditary transthyretin-mediated amyloidosis (hATTR), which is a rare genetic disease marked by abnormal amyloid protein accumulation in peripheral nerves and various organs. The FDA approval of Patisiran in 2018 marked an important advancement for siRNA-based treatments. The therapeutic drug Patisiran uses lipid nanoparticles to transport siRNA to the liver for TTR protein production suppression. The LNP formulation shields the siRNA from breakdown while promoting its absorption by liver cells to achieve precise gene silencing. The specific mechanism of Patisiran reduces unintended side effects and systemic toxicity resulting in a safe and effective therapeutic solution. Patisiran's triumph has opened doors for additional siRNA therapy research while validating the LNPs delivery system.
- Inclisiran: The GalNAc success story
Inclisiran stands as a significant achievement in siRNA research using GalNAc conjugation to deliver treatment specifically to liver cells. The development of GalNAc conjugates has improved siRNA therapy effectiveness by allowing the liver to uptake these treatments more efficiently. The siRNA drug Inclisiran targets the PCSK9 gene responsible for cholesterol regulation, offering potential treatment options for hypercholesterolemia. By using GalNAc conjugation scientists improve siRNA stability and delivery effectiveness which enables subcutaneous delivery and durable effects. Preclinical studies have shown that Inclisiran produces substantial reductions in LDL-C levels. The GalNAc conjugation strategy is now broadly used across multiple nucleic acid therapies such as antisense oligonucleotides (ASOs) and anti-microRNAs (anti-miRs) which demonstrates its broad application potential for disease treatment.
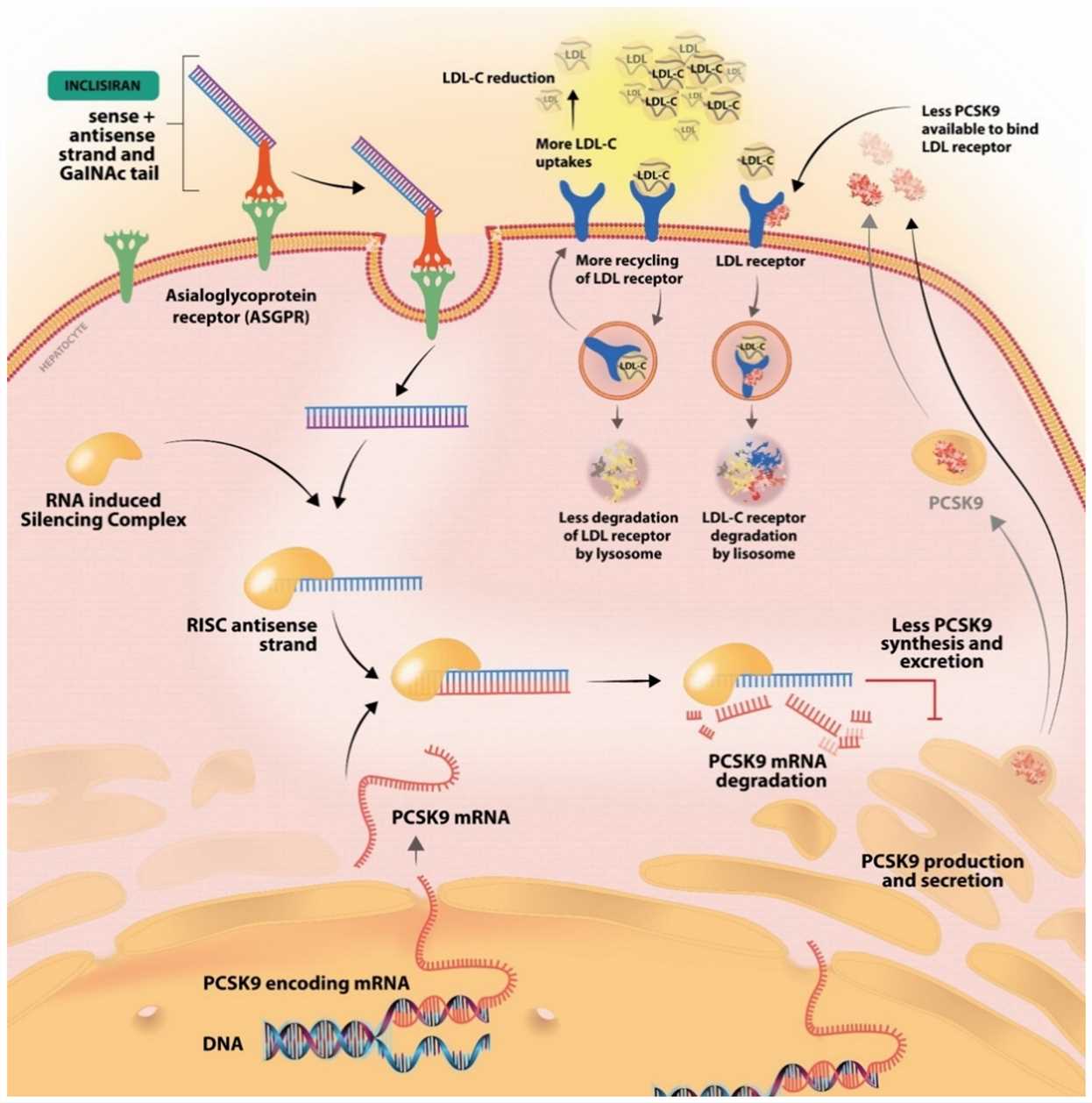 Fig.3 The mechanism of inclisiran, the first synthetic siRNA. After conjugation to GalNac, it binds the ASGPR receptor and is internalized into the cell3,6.
Fig.3 The mechanism of inclisiran, the first synthetic siRNA. After conjugation to GalNac, it binds the ASGPR receptor and is internalized into the cell3,6.
The Future: Delivery-Enabled siRNA Breakthroughs
SiRNA represents RNA therapeutics which targets specific disease-related genes for silencing through the RNAi mechanism. Researchers discovered siRNA-based therapeutics nearly twenty years ago but they found these treatments had multiple limitations. Research studies have explored these challenges and brought hope for future siRNA therapeutics that target any disease through gene-specific silencing. Researchers developed siRNA modifications and alternative delivery methods to improve siRNA delivery effectiveness. Researchers develop nanoparticle-based delivery systems for siRNA to reduce delivery barriers and prevent off-target effects. The delivery systems consist of lipid carriers, polymer nanoparticles, inorganic nanoparticles and an improved siRNA delivery method that avoids toxicity. The LNPs delivery method used for the first FDA-approved siRNA patisiran sparked increased research attention toward the nanoparticle-based delivery approach for siRNA. Cationic liposomes serve as the primary delivery system for siRNA and mask its poly-anionic surface to improve its ability to penetrate membranes. While numerous modifications and nanocarriers have been developed for siRNA delivery, its final bioavailability remains low in the destruction of targeted mRNA to reduce gene expression. Research findings indicate that nanoparticle-based systems for mRNA targeting remain underdeveloped but hold potential for enhanced endosomal escape and reduced off-target effects through the use of targeting ligands.
References
- Wang, Hui, et al. "Design of polymers for siRNA delivery: Recent progress and challenges." View 2.3 (2021): 20200026. https://doi.org/10.1002/VIW.20200026.
- Mirzaei, Sepideh, et al. "Pre-clinical and clinical applications of small interfering RNAs (siRNA) and co-delivery systems for pancreatic cancer therapy." Cells 10.12 (2021): 3348. https://doi.org/10.3390/cells10123348.
- Ilut, Silvina, et al. "Recent advances on the roles of PCSK-9 inhibitors in the management of acute ischemic stroke patients." International Journal of Molecular Sciences 23.18 (2022): 10221. https://doi.org/10.3390/ijms231810221.
- Ebenezer, Oluwakemi, et al. "Development of novel siRNA therapeutics: a review with a focus on inclisiran for the treatment of hypercholesterolemia." International Journal of Molecular Sciences 24.4 (2023): 4019. https://doi.org/10.3390/ijms24044019.
- Tatiparti, Katyayani, et al. "siRNA delivery strategies: a comprehensive review of recent developments." Nanomaterials 7.4 (2017): 77. https://doi.org/10.3390/nano7040077.
- Distributed under Open Access license CC BY 4.0, without modification.
