The Enigmatic World of siRNA-Unveiling the Mechanisms and Potential of Gene Silencing
What is siRNA
Small interfering RNA (siRNA), sometimes referred to as short interfering RNA or silencing RNA functions as a double-stranded RNA molecule that controls gene expression by utilizing the RNA interference (RNAi) pathway. siRNA molecules usually measure between 20 and 25 nucleotides in length. siRNA molecules identify and degrade specific mRNA sequences which stops protein production from those mRNAs. Scientists apply siRNA as a powerful gene silencing instrument to explore genetic functions and create treatment strategies. Researchers create siRNA via direct artificial synthesis or through cellular processing of extended double-stranded RNA molecules by utilizing the Dicer enzyme. siRNA operates with exact precision and efficiency and serves as a fundamental tool in functional genomics research while showing strong promise as a therapeutic agent for treating diseases.
Historical Milestones of siRNA
siRNA technology has reached numerous critical breakthroughs in its development. Scientists identified the RNAi process during their 1998 research on gene expression in Caenorhabditis elegans. Scientists who revealed how double-stranded RNA silences genes earned the 2006 Nobel Prize for their pioneering work. In 1999 researchers announced the finding of siRNAs and their role in post-transcriptional gene silencing within plants. The studies presented fresh understandings of RNA interference mechanisms across various species. In 2001 research showed that specially designed siRNA molecules could trigger RNAi within mammalian cells.
The siRNA Mechanism: A Step-by-Step Guide
- Generation of siRNA
Ribonuclease Dicer processes long double-stranded RNA molecules introduced into mammalian cells through endonucleolytic cleavage to produce siRNAs. The RNase III family endonuclease Dicer functions as a "molecular ruler" which generates RNA duplexes with characteristic termini measuring 21-25 nucleotides in length. A dinucleotide overhang exists at the 3" end while the 5" end finishes in a monophosphate. The RISC requires siRNA duplexes to have specific lengths and unique end structures for proper identification and integration.
- RISC loading
After their formation siRNA molecules get integrated into the multiprotein RISC which plays a crucial role in silencing genetic expression. The RISC complex includes multiple proteins and the Argonaute protein which serves as a pivotal player in the RNAi mechanism. The process of incorporating siRNA into RISC stands as a vital step because it positions the siRNA to properly direct RISC towards its specific mRNA target.
- Strand Selection and Targeting
Within the RISC complex, the siRNA unwinds into two separate strands: the guide strand and the passenger strand. The functional guide strand stays with RISC and guides the complex to target mRNA sequences that are complementary. The targeting process uses base pairing between the guide strand and target mRNA to achieve precise specificity.
- mRNA Degradation
The siRNA mechanism concludes with the target mRNA being degraded. AGO1-4 proteins in RISC target and cleave mRNA at a determined site which results in mRNA degradation. AGO2 needs to bind the guide siRNA strand and remove the passenger strand to perform multiple cycles of target mRNA recognition and cleavage before releasing the degraded mRNA while keeping the guide strand attached. RNAi functions as a robust gene silencing and regulatory tool due to precise siRNA targeting combined with efficient mRNA degradation.
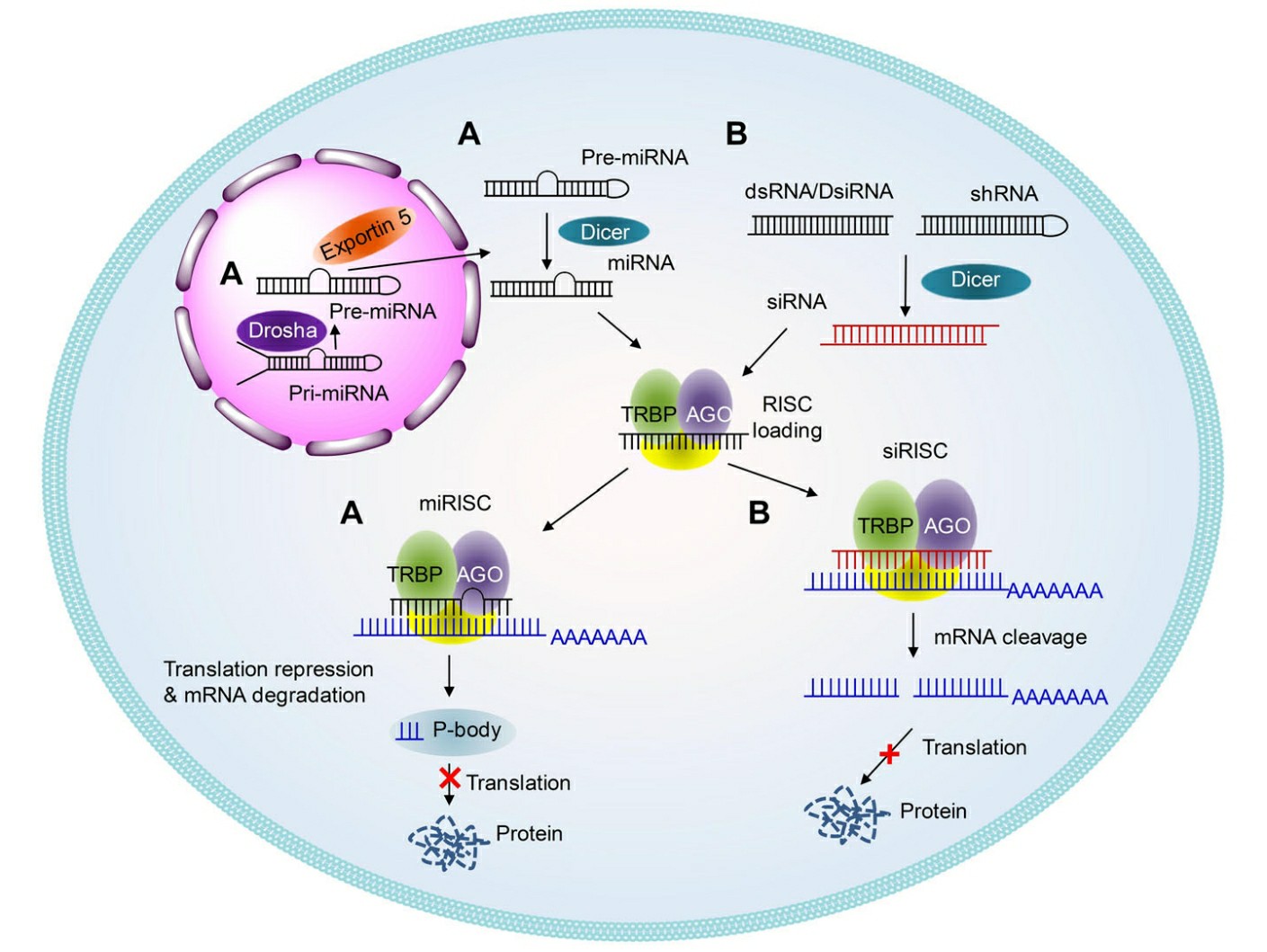 Fig.1 Schematic illustrations of the working mechanisms of miRNA (a) and siRNA (b)1,6.
Fig.1 Schematic illustrations of the working mechanisms of miRNA (a) and siRNA (b)1,6.
siRNA in Cellular Processes
- Regulating Gene Expression
The chemically synthesized siRNA molecule is directly introduced into the cell before it enters the RISC complex which includes multiple proteins such as AGO-2 and Dicer. When the "sense" or "passenger" strand of siRNA gets removed during activation the "antisense" or "guide" strand remains and instructs RISC to bind target mRNA where Ago-2 mediates mRNA cleavage. The RNAi mechanism operates through catalysis unlike antisense technologies because the siRNA-loaded RISC breaks free to bind new mRNA targets after cleaving the initial mRNA. Consequently, siRNA achieves efficient gene silencing at picomolar concentrations and less than 2000 molecules per cell are enough for effective intracellular action.
- Protecting Against Viral Infections
Cells benefit from siRNA because it serves as a critical defense mechanism against viral infections. siRNA provides a new antiviral method by blocking viral replication and spread through targeting viral RNA. SiRNA works by identifying viral RNA sequences and destroying them to block the production of crucial proteins needed for viral replication. Directed siRNAs at conserved viral genome regions demonstrate effective suppression of multiple viruses such as HIV and hepatitis as well as respiratory syncytial virus. Researchers achieve major reductions in viral load by using siRNAs to target viral protein mRNA which helps stop infections from spreading. Quick antiviral treatment development becomes essential for addressing newly emerging viral threats.
- Modulating Cellular Signaling
siRNA serves as a tool to manipulate cellular signaling pathways through the knockdown of essential signaling molecules. The method allows researchers to gain important knowledge about cell regulatory pathways while establishing siRNA as a fundamental instrument in cellular biology studies. Scientists can study the functions of specific proteins in cellular processes like growth and apoptosis by using selective gene silencing techniques on signaling pathway genes. Researchers use siRNA-mediated knockdown of oncogenes or tumor suppressor genes to establish cancer models which help understand tumor biology and identify new therapeutic strategies. siRNA serves as a research tool to investigate the functions of signaling molecules in immune responses as well as metabolic pathways and developmental systems. SiRNA's capability to alter cellular signaling pathways establishes it as an effective instrument for research fundamentals and therapeutic advancements.
siRNA applications in Preclinical Research
- Gene Function Studies
Through targeted gene knockdown with siRNA researchers can examine phenotype changes to understand specific genes' functions in cellular activities. The method demonstrates exceptional precision and effectiveness which establishes it as a foundational element of functional genomics. Serum treatment of myotubes resulted in glucose uptake which showed a connection to higher levels of glucose transporter (GLUT1) on the cell surface. The use of GLUT1 siRNA treatment in cells led to decreased serum-stimulated glucose transport demonstrating GLUT1 expression dependency. A separate research used siRNAs to examine the transient receptor potential canonical 1 (TRPC1) gene believed to produce a non-selective cation channel. TRPC1 siRNA treatment resulted in increased liver cell volume and reduced Ca2+, Mn2+, and ATP inflow under hypotonic conditions which confirmed the initial hypothesis. Researchers use siRNA technology to analyze both oncogenes and tumor suppressor genes. Pancreatic cancer cells treated with siRNA against the KRASG12D oncogene demonstrated reduced rates of cell proliferation and tumor growth. Hepatocellular carcinoma cells undergo cell cycle arrest and apoptosis following siRNA intervention against the PLK1 gene which exhibits overexpression across multiple tumor types.
- Animal Models
Animal model studies have effectively utilized siRNA in the investigation of multiple diseases such as cancer, neurodegenerative disorders and cardiovascular conditions. The research produced important information about how diseases work and new ways to treat them.
- Cancer Research
Researchers utilize siRNA to inhibit oncogenes and genes associated with tumors during cancer studies. Scientists in a mouse breast cancer model study utilized lipid nanoparticles with encapsulated siRNA to attack heparin-binding EGF-like growth factor (HB-EGF) which led to diminished tumor expansion. A multistage vector system delivered siRNA against the EphA2 gene showed reduced tumor cell proliferation and angiogenesis in ovarian cancer models according to a study. The treatment demonstrated substantial tumor growth inhibition while avoiding major toxicity in essential organs.
- Neurodegenerative Disorders
siRNA demonstrates potential benefits in the treatment of neurodegenerative diseases. Intraocular delivery of siRNA against the Lrat gene in a mouse LCA model restored electroretinographic responses to approximately half the level of wild-type mice. The research illustrated how siRNA-based therapies might serve as treatments for genetic blindness.
- Cardiovascular Diseases
Researchers in cardiovascular science have utilized siRNA to silence genes responsible for atherosclerosis and hypertension. Researchers have tested siRNA which targets apolipoprotein A (Apo(a)) in animal models to lower cardiovascular disease risk. Researchers used siRNA to target angiotensinogen (AGT), a critical hypertension factor and achieved significant blood pressure reductions.
- Viral Infections
Researchers have extensively investigated siRNA's antiviral capabilities. The use of siRNA to target viral RNA in a rhesus macaque SARS-CoV infection model demonstrated significant antiviral effectiveness by decreasing viral load and enhancing clinical results. Research showed that siRNA directed against the HBV gene reduced both viremia levels and hepatitis B surface antigen expression in humanized mouse models. Research indicates that siRNA can be an efficient and speedy treatment option for viral infections.
Challenges and Advances in siRNA Delivery
- Delivery Systems
Nucleases enzymes in plasma rapidly degrade injected siRNA as the initial biological barrier to intravascular delivery. Unmodified siRNA lacks stability in systemic circulation because A-type nucleases that exist throughout intracellular and extracellular spaces easily degrade it. Due to rapid renal clearance siRNA exhibits a brief half-life that lasts only between 5 to 10 minutes. Plasma and tissue nucleases rapidly degrade unmodified siRNA within minutes up to an hour which poses a barrier for siRNA therapeutic applications. Their small size which measures about 7 nm long together with their light molecular weight of 13 kDa explains these challenges. The fundamental characteristics of siRNA molecules present significant obstacles to effective delivery into target cells. Due to its strong negative charge siRNA cannot pass through cell membranes easily which prevents it from reaching the cytoplasm where it performs its functions. The siRNA molecule faces degradation by nucleases and risks activating immune responses without adequate protection. Scientists developed multiple delivery systems such as nanoparticles, liposomes, and viral vectors to tackle these problems.
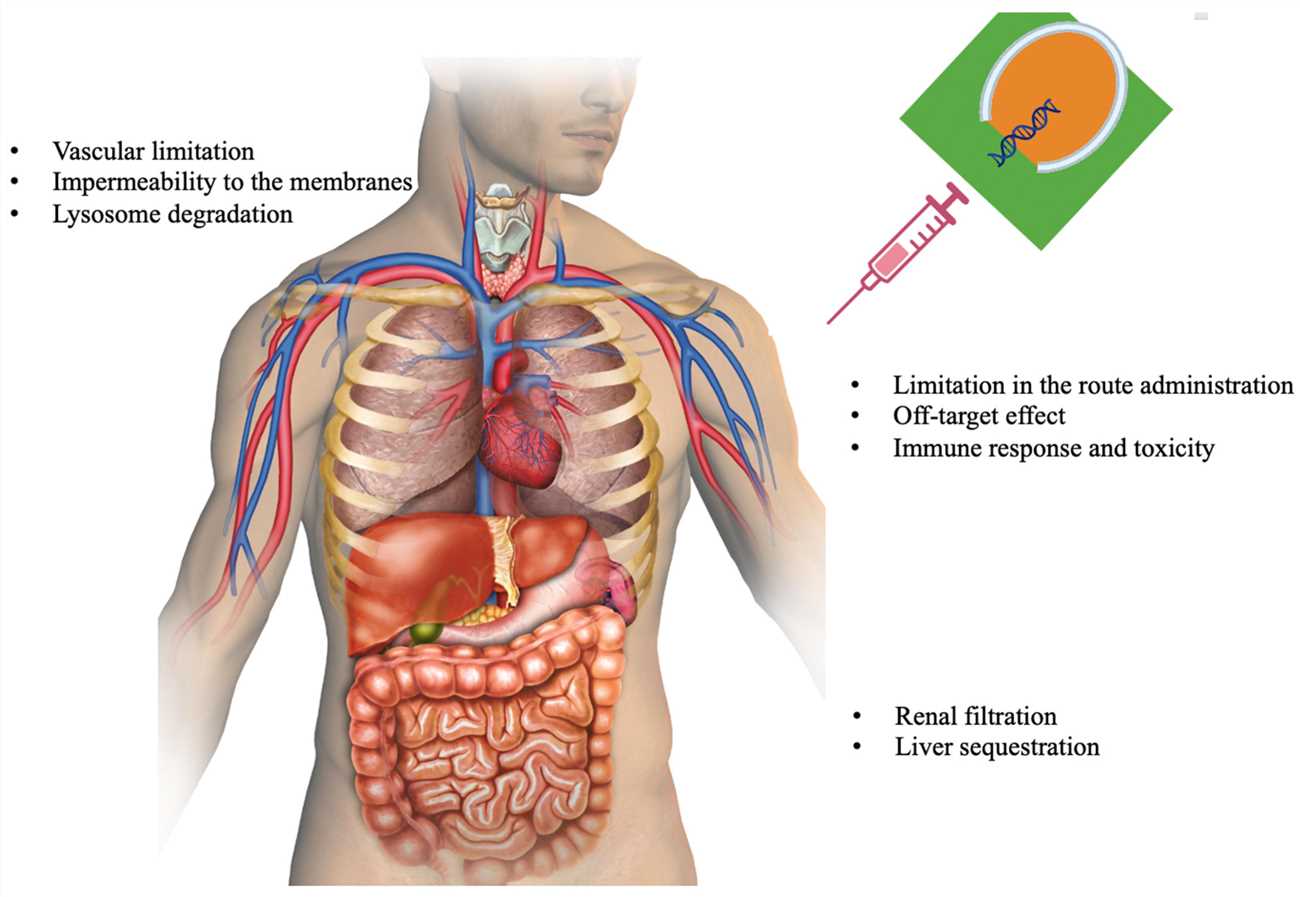 Fig. 2 Challenges to be overcome in the siRNA therapeutics 2,6.
Fig. 2 Challenges to be overcome in the siRNA therapeutics 2,6.
- Nanoparticles
The scientific community now acknowledges nanoparticles as a viable method for transporting siRNA to targeted cells. Nanoparticles as siRNA carriers protect genetic material from breakdown and help it pass through biological membrane barriers. Polymeric nanoparticles constructed from substances like polyethyleneimine (PEI) or chitosan show excellent results when transporting siRNA into specific cells. PEI nanoparticles trigger siRNA endosomal escape via the "proton sponge effect" which leads to improved cellular uptake. Scientists use calcium phosphate nanoparticles to release siRNA in endosomal acidic conditions which enhances delivery efficiency.
- Liposomes
Scientists have extensively investigated cationic liposomes for siRNA delivery across multiple applications. Cationic liposomes create protective complexes with siRNA which prevent degradation and support cellular uptake. LPD nanoparticles consisting of liposome-polycation-DNA complexes successfully delivered siRNA against luciferase achieving substantial gene silencing in mouse models. The addition of ligands such as anisamide to these liposomes enables them to hone in on specific cancer cells which helps reduce unintended effects outside the target area.
- Viral Vectors
Researchers utilize viral vectors such as lentiviruses and adenoviruses to transport siRNA because these vectors provide superior transfection efficiency. Viral vectors activate immune responses and possess restrictions in both safety and their ability to carry siRNA. Scientists are engineering non-viral vectors which replicate the high delivery effectiveness of viral vectors but reduce toxicity and immune response.
- Minimizing Off-Target Effects
The potential for siRNA therapy to cause unintended gene silencing and side effects makes off-target effects a major safety concern. Bioinformatics tools and meticulous siRNA sequence design are essential approaches for reducing unintended effects while increasing target specificity.
- siRNA Sequence Design
The proper design of siRNA sequences proves essential for reducing off-target effects. Researchers select siRNA sequences based on high complementarity to the target mRNA while steering clear of regions with significant sequence similarity to other genes. Chemical modifications to the siRNA structure at the 2'-position of the ribose sugar can improve stability while also decreasing off-target effects.
- Bioinformatics Tools
Scientists created bioinformatics software to identify possible off-target locations and refine siRNA sequences. The tools evaluate siRNA sequences to identify potential genome binding sites and propose sequence modifications to enhance specificity. Algorithms can detect siRNA sequences that show minimal genetic similarity to non-target genes and thus decrease the chance of unintended gene silencing.
- Chemical Modifications
Chemical alterations to siRNA molecules serve to increase both their stability and specificity. Embedding cholesterol or various lipophilic molecules into siRNA enhances its pharmacokinetic profile while minimizing off-target effects. Such modifications increase siRNA's binding with lipoproteins which enables targeted delivery to specific tissues.
Future Directions and Potential Applications
siRNA represents a powerful medical technology that offers substantial prospects for disease treatment across various conditions. The capacity of siRNA to precisely target and silence specific genes makes it a crucial tool for tackling complex diseases such as cancer and viral infections along with neurodegenerative conditions and genetic disorders. Current research efforts continue to work towards creating improved delivery systems for siRNA while investigating the combination of siRNA with other therapies and discovering novel targets by using genomics and computational methods. Through these research initiatives scientists anticipate boosting siRNA's therapeutic effects while broadening its clinical usage. siRNA-based therapies have produced substantial positive results in the treatment of hereditary transthyretin-mediated amyloidosis as well as ocular illnesses including glaucoma. Research teams examine siRNA-based treatments for their potential to fight viral pathogens like COVID-19 and tackle neurodegenerative conditions such as Alzheimer's disease. As scientists improve delivery methods and optimize siRNA molecules the possibility for personalized targeted treatments will expand. Current advancements in medical science promise to revolutionize modern healthcare while simultaneously providing hope to people with diseases that have no known cure. siRNA technology continues to advance and establish itself as a fundamental element for future medical treatments while driving innovative approaches and enhancing patient care.
References
- Hu, Bo, et al. "Therapeutic siRNA: state of the art." Signal transduction and targeted therapy 5.1 (2020): 101. https://doi.org/10.1038/s41392-020-0207-x.
- Ebenezer, Oluwakemi, et al. "Recent Update on siRNA Therapeutics." International Journal of Molecular Sciences 26.8 (2025): 3456. https://doi.org/10.3390/ijms26083456.
- Tatiparti, Katyayani, et al. "siRNA delivery strategies: a comprehensive review of recent developments." Nanomaterials 7.4 (2017): 77. https://doi.org/10.3390/nano7040077.
- Ahn, Insook, Chanhee S. Kang, and Jinju Han. "Where should siRNAs go: applicable organs for siRNA drugs." Experimental & Molecular Medicine 55.7 (2023): 1283-1292. https://doi.org/10.1038/s12276-023-00998-y.
- Hattab, Dima, Amirah Mohd Gazzali, and Athirah Bakhtiar. "Clinical advances of siRNA-based nanotherapeutics for cancer treatment." Pharmaceutics 13.7 (2021): 1009. https://doi.org/10.3390/pharmaceutics13071009.
- Distributed under Open Access license CC BY 4.0, without modification.
