siRNA in Neurodegenerative Diseases-A New Hope for Degeneration
Introduction of siRNA in Neurodegenerative Diseases
Research into small interfering RNA (siRNA) shows potential for treating neurological disorders such as Huntington's disease (HD), Alzheimer's disease (AD), and amyotrophic lateral sclerosis (ALS). Neurodegenerative diseases present with misfolded protein deposits along with neuronal death and advancing deficits in cognitive and motor functions. siRNA provides a precise method for silencing disease-related genes which traditional therapies fail to treat effectively. In HD treatment research siRNA molecules can be engineered to target the mHTT gene directly which results in decreased toxic protein production and a potential slowdown in disease progression. AD research indicates that siRNA directed at amyloid precursor protein (APP) or tau shows potential results in preclinical studies. Because siRNA is capable of targeting and degrading specific mRNA sequences it makes it an effective solution for understanding and treating neurodegenerative diseases on a genetic and molecular level.
siRNA Mechanism in Neurons
- Basic mechanism
Cells trigger the RNAi mechanism through the uptake of double-stranded RNA (dsRNA). The procedure includes two stages starting with an initial phase followed by an effector phase. During the initial phase of RNA interference the enzyme Dicer processes double-stranded RNA into siRNA fragments that measure between 21 and 23 base pairs. At the 3' end of the siRNA molecule two essential nucleotide overhangs exist while its 5' end shows a monophosphate group. The effector stage of RNA interference sees siRNA molecules joining the RNA induced silencing complex (RISC) multiprotein complex. The RISC multiprotein complex facilitates both the completion of siRNA processing and the recognition and digestion of target molecules. Once siRNA joins RISC the antisense strand with the most stable 5' end continues to remain bound to the RISC. AGO operates as the key component and vital effector molecule within RNAi-related gene silencing mechanisms. The guide strand becomes active within the RISC complex when the passenger strand is released. siRNA achieves maximum gene silencing when its guide strand completely matches the mRNA transcript because miRNA targets mRNA for cleavage by RISC's AGO2 component. Cellular nucleases degrade the mRNA following its cleavage process.
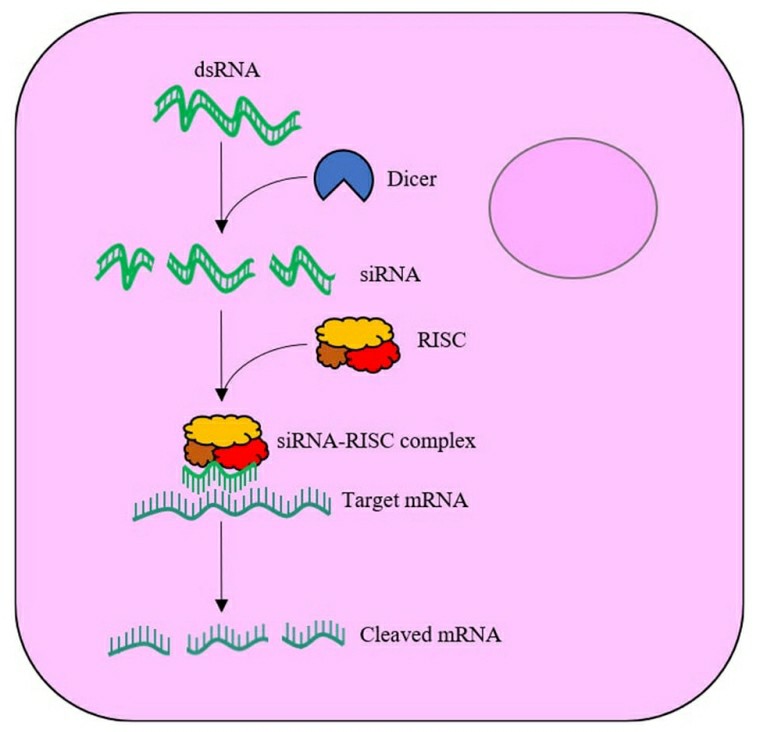 Fig.1 Schematic of the RNA interference (RNAi) mechanism within a cell1,7.
Fig.1 Schematic of the RNA interference (RNAi) mechanism within a cell1,7.
- Targeting Mutant Proteins
The expansion of CAG repeats within the HTT leads to HD which manifests as an autosomal dominant neurodegenerative disorder. The mutant huntingtin protein contains an extended polyglutamine (polyQ) sequence which creates aggregates that damage neurons resulting in their dysfunction and eventual death. The normal huntingtin protein supports synaptic function along with axonal transport and transcriptional regulation but its mutant form interferes with these essential processes. As mHTT builds up inside neurons it creates neuronal intranuclear inclusions (NIIs) and disrupts cellular functions including autophagy and proteostasis.
Scientists can develop siRNA molecules that specifically target mutant HTT mRNA to overcome its toxic effects. Researchers target expanded CAG repeats because they appear exclusively in the mutant allele. Cell culture and animal models demonstrated significant decreases in mHTT protein levels when siRNA molecules targeted the CAG repeats. Researchers use single nucleotide polymorphisms (SNPs) that associate with the mutant allele as a targeting mechanism. Allele-specific silencing protects the necessary wild-type huntingtin protein while targeting only the mutant protein for neuronal health. Selective silencing of mHTT achieved by chemically modified siRNA targeting a specific SNP in the HTT gene has been demonstrated in patient-derived neural stem cells and HD mouse models. By focusing on selective targeting the approach achieves reduced off-target effects and lowers the risk of adverse outcomes from non-specific gene silencing.
- Inhibition of Pathogenic Pathways in Neurons
BACE1 functions as a transmembrane aspartic protease which starts the production of Aβ peptides through its cleavage activity on APP at the β-site. The initial cleavage leads to a C-terminal fragment which γ-secretase processes into Aβ peptides. Plaque formation results from the aggregation of these peptides and serves as a key indicator of Alzheimer's Disease pathology. Research has shown higher BACE1 concentrations in AD patient brains which aligns with greater Aβ production and plaque development. AD therapy requires targeting the reduction of BACE1 activity as a critical objective. Researchers have effectively applied siRNA to reduce BACE1 expression across multiple cell-based and animal models that simulate Alzheimer's Disease. siRNA targets BACE1 mRNA to significantly decrease enzyme levels which leads to reduced Aβ production. Research indicates siRNA targeting BACE1 reduces BACE1 protein levels by 80% in cultured neurons and astrocytes. Reducing BACE1 activity results in substantial decreases in both Aβ40 and Aβ42 levels which represent the primary Aβ forms found in Alzheimer's disease pathology.
Blood-Brain Barrier (BBB) Penetration Strategies
- Focused Ultrasound: Temporary BBB Disruption for siRNA Delivery
Focused ultrasound (FUS) stands out as a promising approach that enables temporary disruption of the BBB to improve brain delivery of therapeutic agents such as siRNA. The FUS method uses concentrated acoustic energy to target precise brain regions which results in temporary BBB disruption in those areas. The combination of microbubbles with ultrasound strengthens mechanical impact on endothelial cells which causes temporary disassembly of tight junctions and increases permeability.
Preclinical investigations have demonstrated effective use of FUS alongside different therapeutic drugs such as siRNA to boost brain delivery. The combination of FUS with siRNA that targets oncogenic drivers in glioblastoma models leads to better siRNA uptake by tumor cells and enhanced therapeutic results. FUS technology has demonstrated effectiveness in Alzheimer's disease models by facilitating brain delivery of therapeutic drugs through the BBB. The safety and efficacy of FUS for BBB disruption in patients with brain tumors and neurodegenerative diseases are being tested through ongoing clinical trials.
- Trojan Horse Liposomes: Transferrin Receptor-Mediated Transcytosis
Scientists design Trojan horse liposomes as nanoparticles which use the BBB's natural transport systems to improve drug delivery across this barrier. The most researched approach includes the application of transferrin (Tf)-decorated liposomes which target the transferrin receptor (TfR) on endothelial cells of the BBB. The liposomes use receptor-mediated transcytosis to pass through the BBB and reach the brain with their molecular payload.
Research has demonstrated that liposomes embedded with transferrin (Tf) enable much stronger delivery of siRNA into brain tissue which results in successful gene silencing within preclinical experiments. Scientists developed solid lipid nanoparticles (SLNs) for siRNA encapsulation that incorporate surface modifications to enable BBB transcytosis via TfR targeting. These Trojan horse liposomes increase siRNA delivery efficiency while also reducing necessary doses which helps to minimize potential side effects.
- Exosomal Delivery: Neuron-Derived Exosomes for Brain Targeting
Cells produce exosomes which researchers can modify to transport therapeutic substances such as siRNA through the BBB. The natural capacity of neuron-derived exosomes to cross the BBB and transport cargo to neurons makes them a particularly promising delivery system. Neuron-derived exosomes can carry siRNA molecules that target specific genes which play a role in neurodegenerative disorders like Alzheimer's disease and Huntington's disease.
Research shows that exosomes from neurons containing siRNA against amyloid precursor protein (APP) successfully decrease Aβ levels in Alzheimer's disease experimental models. Exosomes that contain siRNA which targets mHTT have demonstrated effectiveness in decreasing mHTT levels within Huntington's disease models. Exosomes for siRNA delivery present multiple benefits such as biocompatibility and immune evasion capabilities while enabling precise targeting of specific brain cell types.
Preclinical Studies of siRNA in Neurodegenerative Models
- Animal Models
Animal models in preclinical studies have played a crucial role in determining siRNA therapy effectiveness for neurodegenerative diseases. Transgenic mice expressing human disease genes provide critical insights into the pathological processes of Alzheimer's, Parkinson's, and Huntington's diseases.
Scientists tested siRNA that targets BACE1 using transgenic mouse models. Researchers administered siRNA through lentivirus delivery into the hippocampus of SAMP8 mice which serve as an Alzheimer's disease model and noted a successful reduction in BACE1 expression that resulted in less Aβ accumulation and better cognitive performance. The research utilized siRNA to target BACE1-AS which is a long noncoding RNA responsible for stabilizing BACE1 mRNA. Reduced BACE1-AS expression in AD transgenic mice led to both decreased Aβ levels and enhanced memory performance.
Research demonstrates that siRNA against α-synuclein delivers promising results in multiple animal models of PD. Researchers noted diminished α-synuclein expression and behavioral improvement following α-synuclein siRNA delivery in mice through an AAV viral vector. Researchers found that naked siRNA administered to the substantia nigra region of monkeys decreased α-synuclein levels while producing no harmful effects. Researchers performed siRNA tests on transgenic mice to target the mHTT gene in HD research. In the R6/2 HD mouse model researchers achieved reduced mHTT expression and improved motor function after intraventricular siRNA injection.
- Cell Culture Studies of siRNA
Researchers have used cell culture studies to analyze siRNA mechanisms and refine delivery methods. IMR-32 neuronal cell lines serve as standard models for researching siRNA effectiveness in lowering the expression of proteins that cause diseases.
Scientists have tested siRNA against presenilin-1 (PS1) within IMR-32 neuronal cells. The use of anti-PS1 siRNA in transfection experiments led to significant reductions in Aβ42 levels which shows how siRNA can control Aβ production. Researchers employed siRNA to attack BACE1 in human neuroblastoma cells which led to reduced production of Aβ.
The therapeutic application of siRNA against α-synuclein has undergone evaluation through multiple neuronal cell lines in Parkinson's Disease studies. When naked siRNA was administered to human neuroblastoma cells known as BE(2)-M17 it resulted in decreased α-synuclein levels. The research team applied anionic liposomes with a rabies virus glycoprotein-derived peptide to transport siRNA targeting α-synuclein into primary neuronal cultures which resulted in substantial knockdown.
Advantages of siRNA for the treatment of neurodegenerative disorders
Double-stranded siRNAs have gained widespread application in gene silencing techniques. Upon entering a cell, duplex RNA interacts with the protein components of the RISC complex. Gene silencing synthetic RNAs typically exist as 19–22 base pair duplexes which produce significant translational suppression and decrease target protein levels. The 19–22 bp duplex RNA length ensures stable formation and RISC recognition which produces extended gene suppression effects and eliminates the requirement for frequent dosing. siRNAs that have been chemically modified demonstrate enhanced stability against nucleases which leads to longer periods of action. siRNA-loaded nanocarriers designed for neurodegenerative disease treatment can result in physicochemical changes to siRNA which lower the occurrence of off-target effects. The assistance of either natural or synthetic polymers enables straightforward delivery of substances into the cells. These delivery systems ensure the safety of their contents and enable molecular-level delivery of the payload through established mechanisms. Scientists deem direct siRNA therapy for the human brain unfeasible which drives extensive research toward physicochemical modifications and siRNA encapsulation to improve delivery effectiveness and target-cell specificity.
Future Prospects and Challenges
The quest to discover ideal treatments for neurodegenerative diseases remains an immense challenge because these diseases have complex characteristics and incomplete etiological understanding combined with physiological barriers like the blood-brain barrier that obstruct drug delivery. siRNA shows promise as a therapeutic option for neurodegenerative diseases because it can target specific genes for silencing. Continuous development through in vivo experiments has produced successful new methods for the design, identification and delivery of siRNAs. In vivo proof-of-principle studies have shown that both viral and non-viral delivery techniques enable selective gene targeting and strong suppression with no noticeable toxic effects. The persistent issues with off-target effects remain alongside competition with cellular RNAi components and challenges with effective in vivo delivery. The latest animal model studies indicate that most off-target effects pose no danger however additional critical issues require resolution for RNAi-based drugs to proceed to clinical application. RNAi technology represents a novel therapeutic approach for treating brain disorders today. Multiple human clinical trials using RNAi-based therapies are currently underway. The expectation exists that this technology will demonstrate effective applications in ND.
References
- Pugsley, Charlotte E., et al. "Recent advances in engineered nanoparticles for RNAi-mediated crop protection against insect pests." Frontiers in Agronomy 3 (2021): 652981. https://doi.org/10.3389/fagro.2021.652981.
- Aguiar, Sebastian, Bram van der Gaag, and Francesco Albert Bosco Cortese. "RNAi mechanisms in Huntington's disease therapy: siRNA versus shRNA." Translational neurodegeneration 6 (2017): 1-10. https://doi.org/10.1186/s40035-017-0101-9.
- Upton, Dannielle H., et al. "Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy." Theranostics 12.10 (2022): 4734. https://doi.org/10.7150/thno.69682.
- Ahn, Insook, Chanhee S. Kang, and Jinju Han. "Where should siRNAs go: applicable organs for siRNA drugs." Experimental & Molecular Medicine 55.7 (2023): 1283-1292. https://doi.org/10.1038/s12276-023-00998-y.
- Chernikov, Ivan V., Ulyana A. Ponomareva, and Elena L. Chernolovskaya. "Structural modifications of siRNA improve its performance in vivo." International Journal of Molecular Sciences 24.2 (2023): 956. https://doi.org/10.3390/ijms24020956.
- Tai, Wanyi. "Current aspects of siRNA bioconjugate for in vitro and in vivo delivery." Molecules 24.12 (2019): 2211. https://doi.org/10.3390/molecules24122211.
- Distributed under Open Access license CC BY 4.0, without modification.
