Fibrosis Under Fire-siRNA's Surgical Strike Against Scarring
Introduction to siRNA in Fibrosis
Recent evidence confirms small interfering RNA (siRNA) shows promise as a treatment for fibrosis which occurs due to abnormal extracellular matrix (ECM) accumulation leading to tissue scarring and functional loss. Fibrosis appears regularly as a pathological condition in various organs including the lungs, liver, heart and kidneys. siRNA uses RNA interference (RNAi) to target and silence fibrosis-promoting genes enabling precise adjustments to disease pathways. RNAi stands as a sophisticated approach to selectively silence genes using siRNAs that measure between 21 to 23 nucleotides. Once target cells receive the transfection ATP-dependent helicase separates double-strand siRNAs enabling functional antisense strands to enter the RNA-induced silencing complex (RISC) which then binds target mRNAs to trigger their degradation. Hereditary transthyretin-mediated amyloidosis received its first siRNA-based treatment when the FDA approved patisiran in early 2018 using lipid nanoparticle delivery. Clinical trials now feature several siRNA-based treatments with one example being the lipid nanoparticle containing HSP47 siRNA developed to target liver fibrosis.
Introduction: The Silent Epidemic of Fibrosis
- Fibrosis as a Deadly Common Thread
Many chronic conditions that affect the lungs as well as the liver heart and kidneys share fibrosis as their deadly fundamental cause. Hepatic fibrosis progression in the liver results in cirrhosis which continues to be a major cause of sickness and death worldwide. IPF causes progressive lung scarring which leads to respiratory failure in patients. The activation of specific tissue cells leads hepatic stellate cells in the liver and lung fibroblasts to overproduce ECM proteins like collagen causing fibrosis pathophysiology development.
- Current Treatments
Present fibrosis treatments work through palliative care approaches that aim to control symptoms and slow the progression of the disease instead of offering a permanent cure. Antiviral treatments help slow down liver disease progression in hepatic fibrosis patients since no antifibrotic therapies have received regulatory approval. The medications pirfenidone and nintedabine can slow down IPF progression yet they fail to reverse established fibrosis. The current treatment limitations underscore the critical demand for precision therapies that specifically target fibrosis' molecular mechanisms.
- siRNA's Promise: A Molecular Scalpel to Halt Scarring at Its Genetic Roots
Through its action as a genetic-based precision tool siRNA shows potential to stop scarring directly at its DNA foundation. siRNA reduces ECM protein production while stopping fibroblast and stellate cell activation through targeted silencing of fibrosis-related genes. Research has demonstrated that siRNA which targets the collagen-specific chaperone heat shock protein 47 (HSP47) can successfully reverse liver fibrosis in animal models. Studies have confirmed that siRNA which targets UHRF1 effectively reduces lung fibrosis through fibroblast activation inhibition.
siRNA's Battle Plan: Precision Targets in Fibrosis
- Target TGF-β siRNA-Silencing the master regulator of fibrosis
Studies show that TGF-β1 to TGF-β3 from the TGF-β group are associated with fibrosis development with liver fibrosis as a prominent target. Research findings show that TGF-β1 suppression using siRNA decreases α-SMA and type I collagen levels in HSC-T6 cells and produces antifibrotic effects in mice and rats treated with carbon tetrachloride. Researchers showed how induced BMP-7 expression through a recombinant adenovirus vector produced antifibrotic effects in rat and human HSCs and a thioacetamide-induced fibrotic rat model because TGF-β1 and BMP-7 have opposing roles in liver fibrosis progression. Gremlin 1 functions as a target protein which becomes upregulated with TGF-β1 expression and acts both as a phosphorylating agent for SMAD2/3 within TGF-β signaling and as a BMP-7 inhibitor. Knockdown of gremlin1 using siRNA leads to suppressed HSC activation and reduced liver fibrosis progression in rats exposed to CCl4.
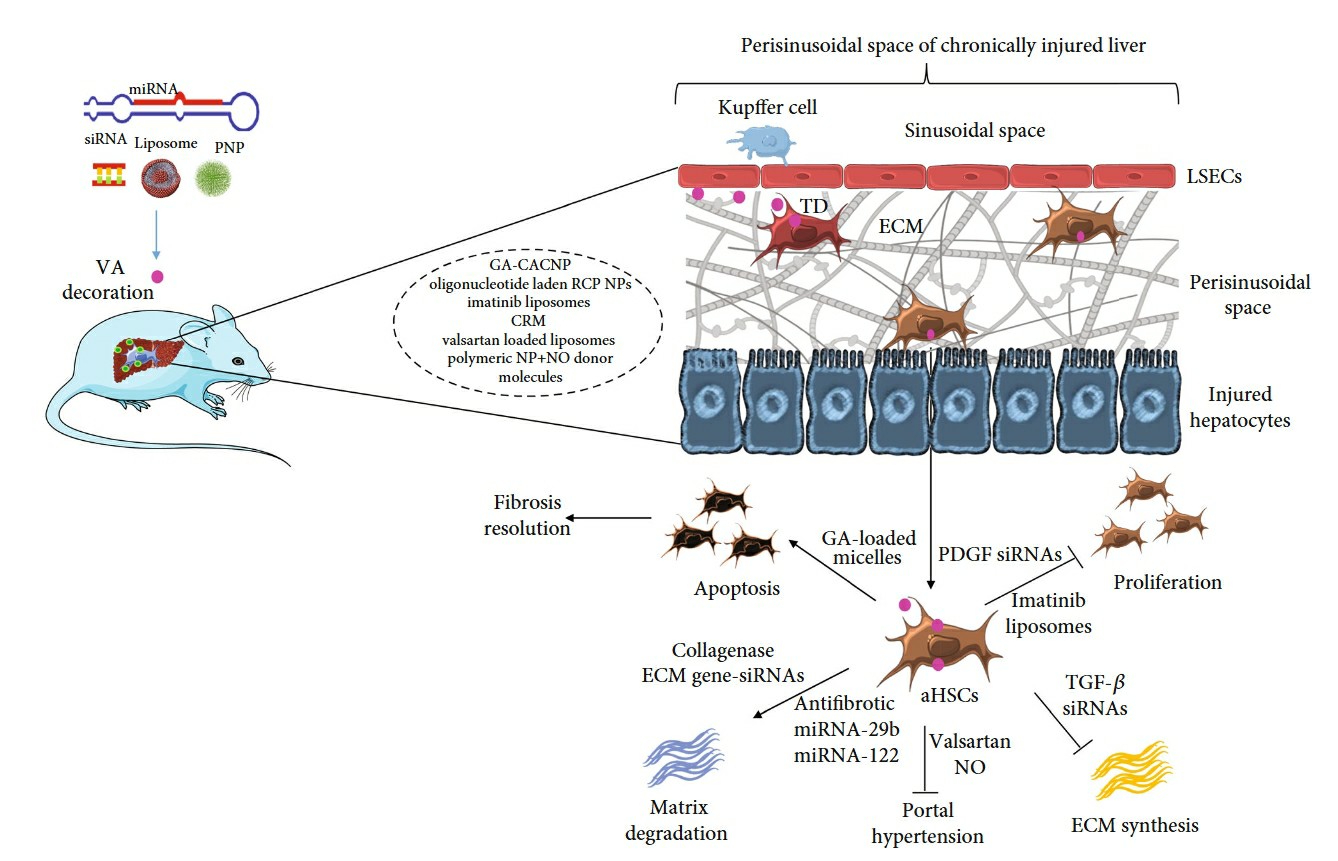 Fig. 1 Targeted drug delivery for activated HSC (HSCs) in the fibrotic liver1,6.
Fig. 1 Targeted drug delivery for activated HSC (HSCs) in the fibrotic liver1,6.
- Target COL1A1 siRNA-Halting runaway collagen production
Activated HSCs contain type I collagen as their most plentiful ECM component which originates from the COL1A1 and COL1A2 genes. The direct regulation of collagen expression in HSCs through siRNA application has successfully treated liver fibrosis in both laboratory and real-life settings. Scientists created a lipid-like nanoparticle carrier for COL1A1 siRNA which successfully suppressed type I collagen production in the human HSC cell line LX-2 and CCl4-treated fibrotic mice livers while avoiding activation of innate immune responses. A lipid-like nanoparticle connected to vitamin A enabled COL1A1 siRNA transport, which showed substantial collagen production inhibition without severe adverse effects when administered at 3 mg siRNA/kg. Scientists created a cationic nanohydrogel particle for COL1A1 siRNA delivery to treat mice with CCl4-induced liver fibrosis. The cationic nanohydrogel particles achieved a 70% reduction of COL1A1 mRNA in fibroblasts after 48 hours of laboratory transfection and also decreased COL1A1 mRNA by 50% in the livers of mice with liver fibrosis.
- Target TIMP-1 siRNA-Freeing matrix metalloproteinases (MMPs)
MMPs and their inhibitors TIMPs control matrix degradation activities which are crucial for both advancing and reversing liver fibrosis. Following liver injury activated HSCs synthesize large quantities of types I and III collagens while simultaneously enhancing TIMP secretion that blocks MMP-regulated ECM degradation. Studies have shown that the expression of MMPs in the liver drives both the development of fibrosis and the reversal of liver tissue damage. Activated HSCs expressed MMP-2 and MMP-14 at high levels which led to increased migration, proliferation, and activation of HSCs. The delivery of a vitamin A-coupled liposome carrying MMP-2-specific siRNA led to a significant decrease in type I collagen and α-SMA expression levels in HSC-T6 cells. TIMP-1 stands out as the main inhibitor of MMPs and its levels increase in response to multiple cytokines during liver fibrosis development. A recombinant adeno-associated virus carrying TIMP-1 siRNA produced antifibrotic effects in both activated HSCs and fibrotic rats induced by CCl4 and bile duct ligation. The combination of siRNAs against TIMP-1 with those targeting connective tissue growth factor demonstrated greater effectiveness in preventing liver fibrosis in rats at risk of developing cancer.
- Target Nuclear Factor-Kappa B and advanced glycation end products (RAGE)
NF-κB signaling influences numerous biological processes including immune response, inflammation regulation, cell death, embryonic growth patterns and disease and cancer formation. The NF-κB signaling pathway operates under regulation through interactions with IκB family proteins and both IκB proteins and IκB kinase complex. The receptor for RAGE engages with its ligands which boost receptor expression to initiate NF-κB pathway activation. Studies have shown that activated HSCs exhibit a significant surge in RAGE levels. The research focused on identifying how RAGE-specific siRNA functions and its role in suppressing liver fibrosis within a rat model. RAGE-specific siRNA treatment slowed liver fibrogenesis progression and decreased inflammatory activity through regulation of the NF-κB signaling pathway. The silencing of RAGE lead to decreased expression levels of both α-SMA and type I collagen.
siRNA Delivery in fibrotic tissue: The Trojan Horse Challenge
- Barriers: Degradation, Off-Target Effects, Reaching Fibrotic Tissue
Multiple significant barriers emerge during the delivery process of siRNA into fibrotic tissue areas. siRNA molecules degrade rapidly because endonucleases found in biological fluids break them down without protection. When siRNA degrades it reduces its therapeutic impact and triggers immune responses that lead to undesirable side effects. siRNA molecules cannot cross cell membranes because their negative charge and hydrophilic nature make this process impossible. The unintended silencing of non-target genes by siRNA represents a significant issue which results in unexpected effects. Therapeutic agents encounter significant difficulties when trying to access fibrotic tissue because of its dense extracellular matrix combined with changed vascular permeability in these regions.
Innovative Solutions
- Lipid Nanoparticles (LNPs)
Lipid nanoparticles represent a promising delivery method for siRNA which shows particular effectiveness in targeting liver tissues. LNPs shield siRNA from degradation while enabling its delivery directly to hepatocytes. In non-alcoholic steatohepatitis (NASH) trials LNPs functioned as delivery carriers for siRNA molecules that target specific genes associated with liver fibrosis. LNPs deliver siRNA to cells through membrane fusion which releases genetic material into the cytoplasm to achieve gene silencing. Research shows that LNPs enhance siRNA stability and delivery effectiveness which makes them powerful tools for liver fibrosis therapy.
- Peptide-Conjugated siRNA: Homing to Lung or Heart Fibroblasts
The development of peptide-conjugated siRNA provides another advanced method to address delivery hurdles. Scientists direct siRNA to lung and heart tissues via fibroblast-specific peptides attached to the siRNA molecule. Cyclic RGD peptides serve as targeting molecules for integrin receptors which fibroblasts in fibrotic tissues express in excess. Directing siRNA to fibroblasts enhances gene silencing and reduces fibrosis due to better uptake.
- Hydrogel Slow-Release: For Localized Delivery
Hydrogel delivery systems serve as a distinctive platform for siRNA administration to specific locations especially when treating post-surgical adhesions. Engineered hydrogels deliver siRNA at a gradual pace to maintain long-lasting gene silencing directly at the intended site. Localized delivery of siRNA through controlled release systems effectively prevents fibrosis and surgical adhesion formation by inhibiting fibroblast activation which leads to decreased tissue scarring.
siRNA in Action: preclinical Progress and Trials
- Liver Fibrosis: ALN-HBV
ALN-HBV as a siRNA therapy is designed to address liver fibrosis caused by hepatitis B virus infection. The hepatitis B virus infection develops into chronic hepatitis and later advances to cirrhosis before turning into liver cancer. ALN-HBV treatment employs RNA interference techniques to block viral gene expression which reduces viral replication and inflammation and prevents fibrosis. Preclinical investigations have demonstrated that ALN-HBV significantly decreases HBV DNA and HBsAg surface antigen levels which represent critical indicators of viral function and liver tissue injury. This precise treatment strategy can both stop liver fibrosis from advancing and enhance patient prognosis with chronic hepatitis B.
- Lung Fibrosis
ND-L02-s0201 serves as an siRNA treatment that suppresses transforming growth factor-beta (TGF-β), a vital cytokine in lung fibrosis development. TGF-β stimulates fibroblast activation which causes extracellular matrix protein buildup that creates IPF tissue scarring. The therapeutic effectiveness of ND-L02-s0201 is achieved by silencing TGF-β which results in reduced fibroblast activation and collagen production for lung fibrosis treatment. Preclinical research demonstrates that ND-L02-s0201 reduces lung tissue stiffness and enhances respiratory function suggesting its potential as an effective IPF treatment option.
- Cardiac Fibrosis
Researchers use siRNA to target Connective Tissue Growth Factor (CTGF) as a potential treatment strategy for cardiac fibrosis. CTGF functions as the main component responsible for activating fibroblasts and accumulating collagen in the heart which leads to fibrosis development and heart failure. Application of siRNA to CTGF silencing blocks fibroblast proliferation and stops collagen formation thereby reducing cardiac fibrosis and enhancing heart function. Animal model studies demonstrate siRNA treatment results in reduced cardiac fibrosis and better heart performance when applied to heart disease models.
Beyond Fibrosis: siRNA's Expanding Horizons
- Dupuytren's Contracture: Silencing Collagen in the Palm
Dupuytren's contracture (DD) develops as a fibrotic disorder within the palmar fascia which leads to progressive finger joint contractures. Patients who undergo surgery and collagenase injections or radiation therapy for Dupuytren's contracture generally encounter suboptimal outcomes as their disease symptoms commonly return. The latest studies focus on using siRNA to inhibit essential cytokines and growth factors linked to DD including TGF-β1 and TNF. Research demonstrates that TNF-targeted neutralizing antibodies reduce collagen type I and alpha-smooth muscle actin (α-SMA) expression in palmar fibroblasts leading to diminished contracture. The specific gene silencing methodology represents a potential breakthrough for DD treatment by disabling the genes that cause fibrosis.
- Keloid Scars: A Cosmetic and Therapeutic Breakthrough
The dermal fibrotic tumors known as keloid scars present benign characteristics through their excessive collagen buildup and fibroblast expansion. Standard treatments such as corticosteroids surgery and radiation show limited results and lead to high recurrence rates for patients. Modern research has investigated siRNA therapy directed at autocrine motility factor (AMF) due to its excessive expression in keloid fibroblasts. AMF siRNA treatment effectively stops keloid fibroblast growth and migration while diminishing collagen levels. Hypersilencing AMF siRNA in mouse keloid transplantation models led to smaller keloids and softer fibrous tissue indicating AMF siRNA as a promising treatment method.
The effectiveness of pirfenidone as an antifibrotic drug increases when used alongside siRNA in combination therapy. Pirfenidone acts as an antifibrotic medication which slows down idiopathic pulmonary fibrosis progression by blocking the TGF-β pathway. New research has investigated using pirfenidone together with siRNA that targets important fibrotic genes such as TGF-β1 and CTGF. The combination treatment method seeks to deliver better therapeutic outcomes through simultaneous attacks on various fibrotic pathways.
References
- Ezhilararasan, Devaraj, Thangavelu Lakshmi, and Biond Raut. "Novel Nano‐Based Drug Delivery Systems Targeting Hepatic Stellate Cells in the Fibrotic Liver." Journal of Nanomaterials 2021.1 (2021): 4674046. https://doi.org/10.1155/2021/4674046.
- Ruigrok, Mitchel JR, et al. "Gene therapy strategies for idiopathic pulmonary fibrosis: recent advances, current challenges, and future directions." Molecular therapy Methods & clinical development 20 (2021): 483-496. https://doi.org/10.1016/j.omtm.2021.01.003.
- Wang, Fa-Da, Jing Zhou, and En-Qiang Chen. "Molecular mechanisms and potential new therapeutic drugs for liver fibrosis." Frontiers in pharmacology 13 (2022): 787748. https://doi.org/10.3389/fphar.2022.787748.
- Kaps, Leonard, and Detlef Schuppan. "Targeting cancer associated fibroblasts in liver fibrosis and liver cancer using nanocarriers." Cells 9.9 (2020): 2027. https://doi.org/10.3390/cells9092027.
- Fu, Rao, et al. "Administration of a combination of COX-2/TGF-β1 siRNAs induces hypertrophic scar fibroblast apoptosis through a TP53 mediated caspase pathway." Scientific Reports 14.1 (2024): 26427. https://doi.org/10.1038/s41598-024-77756-1.
- Distributed under Open Access license CC BY 4.0, without modification.
