Application of gene therapy in cancer treatment- From theory to preclinical research
The importance of gene therapy in cancer treatment
The increasing incidence and mortality rates of cancer worldwide create a major public health crisis. According to the World Health Organization (WHO) cancer holds the position of the second leading cause of death worldwide and resulted in around 10 million deaths across the globe during 2020. Surgery alongside radiation and chemotherapy form the main cancer treatment strategies yet they often lead to severe side effects and fail to treat advanced or refractory cancer types. The last few years have shown an increasing potential for gene therapy to be used in cancer treatment. Gene therapy operates by delivering genetic material into host cells to address or lessen disease symptoms. Cancer gene therapy approaches involve both the direct delivery of therapeutic genes to target cells by means of viral vectors like adenovirus and AAV or non-viral vectors and methods to control immune responses of tumor cells or host immune systems along with manipulating tumor microenvironments to decrease tumor blood vessel formation or enhance tumor antigen presentation to boost immune system detection of tumor cells.
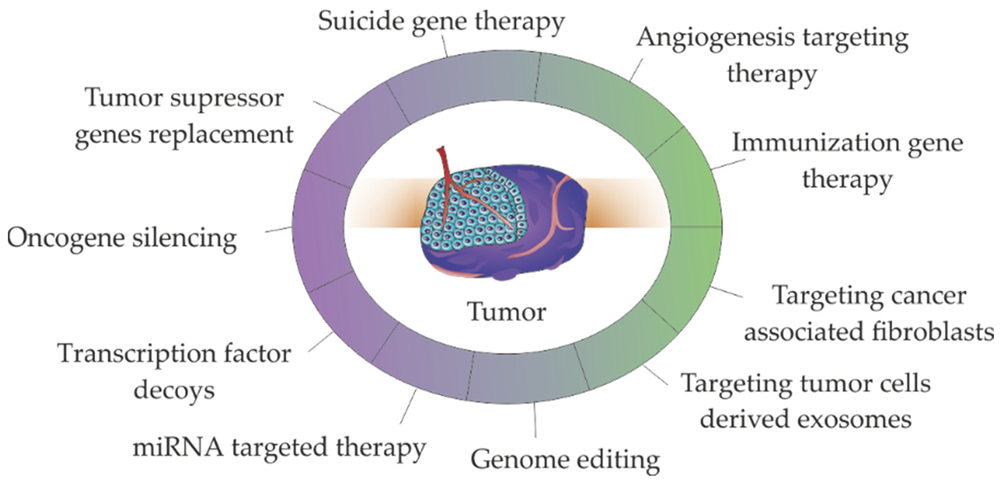 Major strategies used in non-viral gene therapies for cancer treatment1,6.
Major strategies used in non-viral gene therapies for cancer treatment1,6.
Cancer treatment strategies for gene therapy
- Gene repair
Gene repair represents a gene therapy approach which employs gene editing methods to correct mutated genes within cancer cells. CRISPR-Cas9 technology functions as a precise tool for finding and correcting genetic mutations within cancer cells. The technique yields encouraging outcomes across different cancer models with particular effectiveness in fixing tumor suppressor genes. Research efforts are currently focused on repair strategies that target p53 gene mutations in order to restore the function of this crucial tumor suppressor protein which is commonly deactivated in multiple cancer types.
- Immunotherapy
Immunotherapy uses gene therapy methods to strengthen cancer-targeting immune cells by employing platforms like CAR T cells. CAR T cell therapy stands out as a revolutionary treatment method which genetically modifies T cells to express chimeric antigen receptors enabling them to identify and destroy cancer cells. The FDA approved CAR T cell treatments for both acute lymphoblastic leukemia (ALL) and large B-cell lymphoma. Researchers aim to improve immunotherapy results by refining T cell receptors in TCR-T cell therapy for superior cancer antigen identification.
- Suicide gene therapy
Researchers use suicide gene therapy to insert specific genes into cancer cells which enable them to react to certain pharmaceutical drugs. The therapeutic approach includes introducing a gene that produces an enzyme which transforms a harmless prodrug into a harmful substance in cancer cells. Upon administration of prodrugs to patients cancer cells transform these substances into toxins that kill cancer cells. Preclinical studies demonstrate this approach produces promising outcomes through decreased tumor size and extended survival rates.
- Small RNA technology
Small RNAs like siRNA and miRNA serve as cancer therapy tools by blocking oncogene expression and controlling apoptosis mechanisms. siRNA functions to silence specific oncogenes which leads to the inhibition of tumor cell growth and proliferation. The miRNAs control cell growth and death through the regulation of genetic expression networks. Small RNA technologies demonstrate significant therapeutic promise across different cancer models through their use in combination therapy approaches.
Breakthrough in preclinical research
- Animal model study
Mice and rat models remain fundamental for preclinical studies that evaluate the therapeutic impact and safety aspects of gene therapy in cancer treatment research. Researchers have found that AAV vector-mediated gene therapy shows both promising therapeutic outcomes and safety across various animal models. The research verifies gene therapy's effectiveness while creating necessary data for upcoming clinical trials. Animal model studies were performed by researchers to examine long-term treatment outcomes and identify possible side effects to optimize therapy.
- Cell assay
The use of in vitro cell experiments plays a crucial role in gene therapy research through their application within cancer cell lines. Researchers utilize these experiments to assess how gene therapy suppresses cancer cell growth and triggers cell death through apoptosis. Gene-editing technology enables the restoration of normal cellular function in cancer cells by repairing their mutated genes which results in the inhibition of tumor growth. Scientists utilize in vitro experiments to verify both the effectiveness and precision of various gene therapy approaches while generating fundamental information needed for preclinical studies.
- Combination treatment strategy
Present studies focus on combining gene therapy with conventional cancer treatments such as chemotherapy and radiotherapy. The combination treatment strategy aims to improve therapeutic results and minimize side effects by taking advantage of multiple therapies' synergistic interactions. Combining gene therapy with chemotherapy drugs improves treatment effectiveness by augmenting cancer cell sensitivity to chemotherapy treatments. Researchers are using gene therapy alongside radiation therapy to heighten the responsiveness of tumor cells to radiation which improves therapeutic outcomes. Preclinical studies demonstrated effective synergy between combined treatment strategies which informs new clinical application possibilities.
The challenges of gene therapy in cancer treatment
- Tumor heterogeneity
Tumor cell heterogeneity describes how different cells within the same tumor exhibit distinct genotypes and phenotypes which critically influence the outcome of gene therapy treatments. Tumor cell heterogeneity creates treatment resistance because various cells react differently to therapies. Despite gene therapy's ability to correct or inhibit certain genes in some cancer cells, cancer cells with distinct mutations or gene expression patterns remain capable of proliferation and dissemination. The diversity within tumor microenvironments influences gene therapy outcomes and creates varying delivery efficiencies for gene therapy vectors based on differing conditions.
- Immune escape
A major hurdle for gene therapy stems from cancer cells avoiding immune detection through various mechanisms. Cancer cells can decrease their antigen expression to prevent immune cell detection. Cancer cells achieve immune escape during CAR-T cell therapy by decreasing their expression of targeted antigens like CD19 or by losing them entirely. The presence of immunosuppressive elements such as regulatory T cells and cytokines within the tumor microenvironment may weaken the antitumor effectiveness of gene therapy.
- Long-term efficacy and safety
Evaluating the long-term effectiveness alongside the safety profile of gene therapy for cancer treatment represents a critical concern. Clinical trials involving gene therapy have documented late-onset adverse events including the development of cancer because of insertion mutations. The emergence of cancer diagnoses in two sickle cell disease trial participants five years after gene therapy treatment has highlighted concerns about the long-term safety of gene therapy. Further research is needed to determine the effectiveness of gene therapy over extended periods since immediate treatment results do not necessarily ensure stable long-term effects.
Future development of gene therapy in cancer treatment
- Developing new gene editing tools
CRISPR-Cas9 gene editing technology enables precise correction of mutated genes present in cancer cells. Targeted knockout of tumor immune checkpoint molecules can boost immune cell recognition and destruction of cancer cells. In vivo CRISPR screening functions as a critical tool to identify the molecular networks and signaling pathways that control inhibitory immune checkpoint molecules. Simultaneous targeting of multiple antigens represents a valid approach for combating immunogenic heterogeneity. The approach reduces immune escape risks by targeting multiple antigens when heterogeneous immunogenicity causes antigen loss in solid tumors. Preclinical studies have been conducted to test anti-CD38 /BCMA CAR T cells in multiple myeloma models.
- Combined use of immune checkpoint inhibitors
The immune checkpoint inhibitors anti-PD-1, anti-PD-L1, and anti-CTLA-4 work by blocking ligand binding to remove immune suppression and activate immune cells against tumors. The combination treatment of LAG-3 inhibitors with PD-1 inhibitors significantly improved progression-free survival in preclinical melanoma models. Research in the future will concentrate on creating new immune checkpoint inhibitors that will address various immune escape mechanisms. Clinical trials demonstrate the potential effectiveness of combined treatment approaches focusing on TIGIT and PD-1.
Gene therapy holds significant potential for future cancer treatment applications
- Personalized treatment
The use of spatial omics along with single-cell sequencing and liquid biopsy enabled a complete analysis of intratumor heterogeneity and the immune microenvironment for developing personalized treatment plans. The ICRAFT platform enables researchers to systematically discover essential genes with immunomodulatory roles in both tumor cells and immune cells which aids in developing enhanced immunotherapy techniques. The research group created a novel personalized brain tumor organoid model (IPTO), built an organoid bank and proved better accuracy in predicting patient responses to drugs.
- New combination treatment options
Gene therapy will be used alongside chemotherapy and radiotherapy as well as immunotherapy and other treatments to create enhanced therapeutic outcomes. Tumor adaptive evolution becomes delayed through early treatment interventions or the use of intermittent administration and sequential therapy. The development of multi-target vaccines for sub-clonal neoantigens along with immune checkpoint inhibitors will achieve "full coverage" killing and represents a future development direction.
References
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P. V. Gene therapy in cancer treatment: why go nano?. Pharmaceutics. 2020, 12(3): 233. https://doi.org/10.3390/pharmaceutics12030233.
- Bairqdar, A.; Karitskaya, P. E.; Stepanov, G. A. Expanding Horizons of CRISPR/Cas Technology: Clinical Advancements, Therapeutic Applications, and Challenges in Gene Therapy. Int. J. Mol. Sci. 2024, 25(24): 13321. https://doi.org/10.3390/ijms252413321.
- Hidai, C.; Kitano, H. Nonviral gene therapy for cancer: a review. Diseases. 2018, 6(3): 57. https://doi.org/10.3390/diseases6030057.
- Áyen, Á.; Jimenez, Martinez. Y.; Marchal, J. A. Recent progress in gene therapy for ovarian cancer. Int. J. Mol. Sci. 2018, 19(7): 1930. https://doi.org/10.3390/ijms19071930.
- Jogalekar, M.P.; Rajendran, R. L.; Khan, F. CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front. Immunol. 2022, 13: 925985. https://doi.org/10.3389/fimmu.2022.925985.
- Distributed under Open Access license CC BY 4.0, without modification.
